PITTSBURGH, Pa. (Ivanhoe Newswire) – Spinal stenosis is a narrowing of the spinal canal that puts pressure on the spinal cord and nerves. It can cause debilitating pain and numbness in the lower back and legs, and 10 percent of all Americans will experience it. Patients now have a new FDA-approved treatment option that is helping them become pain-free without fusion.
Nathan Snyder has been an athlete his entire life—he played football at Harvard, but his back pain started even before college. Little by little, the pain got the best of him.
“As it progressed, it started as nerve pain down my leg and it eventually got all the way to my toes on both sides,” he said.
Snyder was diagnosed with spinal stenosis – a wear and tear of the joints in the lower spine, creating bone spurs that push on the nerves.
“I stopped running – consistently running seemed to trigger it. I had to stop playing basketball,” he said.
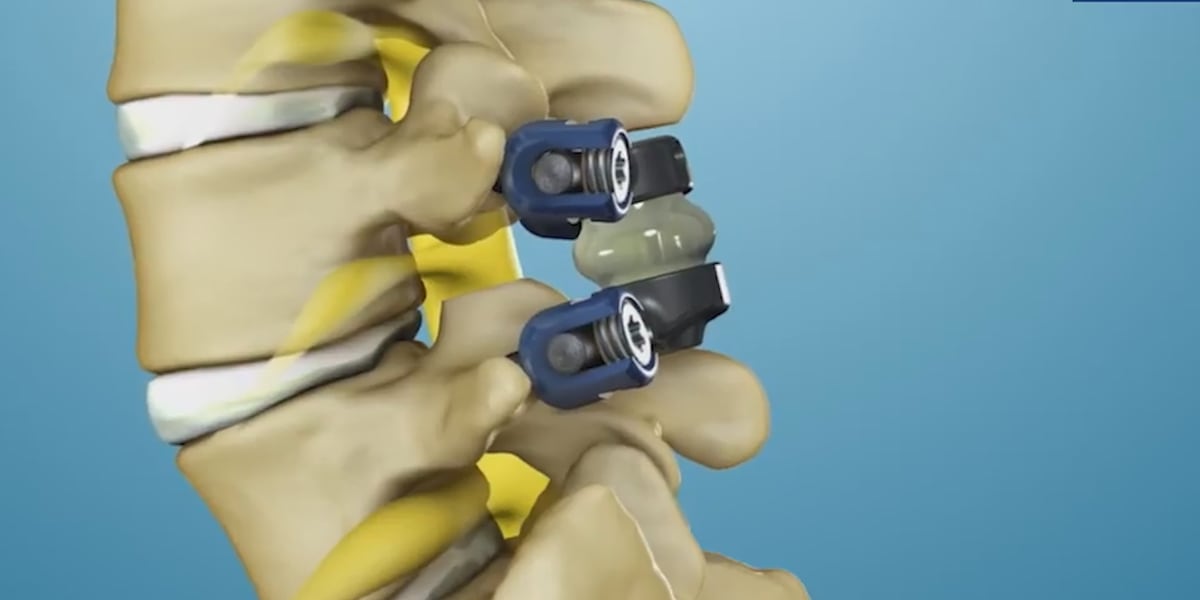
Dr. Donald Whiting, the chair of neurosurgery at Allegheny Health Network, offered Snyder an alternative to spinal fusion that would relieve his pain and preserve his range of motion. The procedure is called TOPS posterior arthroplasty. Surgeons alleviate nerve pressure by substituting bone with two movable titanium joints, and they do not insert a rigid rod – typical of a spinal fusion.
“There’s less wear and tear on the levels above and below and less a need for further surgery down the road,” Dr. Whiting explained.
Snyder said he knew immediately it had worked. Now, he’s pain-free, can bend, flex, walk, workout – do all the things he couldn’t do for decades.
“I feel better than I did at 30,” he said.
Snyder was part of a clinical trial, but now, the TOPS device is FDA-approved and can be used for patients with problems in the spine from vertebrae L3 to L5, the segments of the spine most commonly affected by spinal stenosis.
Click here to report a typo.
Copyright 2024 WAFB. All rights reserved.