Abstract
Oxytocin (OXT) is a neuropeptide hormone termed “love hormone” produced and released during childbirth and lactation. It is also produced in response to skin stimulation (e.g., during hugging and massaging) and music therapy. The effects of OXT on various organs have been revealed in recent years; however, the relationship between hair follicles and OXT remains unclear. In this study, we examined the effects of OXT on dermal papilla (DP) cells that control hair growth by secreting growth/regression signals. Gene expression analysis revealed that DP signature markers were significantly upregulated in DP cells treated with OXT. In addition, we tested the hair growth-promoting effects of OXT using in vitro hair follicle organoids. OXT promoted the growth of hair peg-like sprouting by upregulating the expression of growth-promoting factors, including genes encoding vascular endothelial growth factor A (VEGFA). This study highlights the positive effects of OXT in hair follicles and may assist in the development of new treatments for alopecia.
Introduction
People of all ages and sexes are at risk of hair loss caused by genetics, stress, health conditions, and medicines1, 2. Current treatments for hair loss include drug therapy3, hair transplantation4, and stem cell transplantation5. Drug therapy is commonly used but is limited by poor treatment outcomes and side effects6. Therefore, developing more effective drugs with fewer adverse side effects is crucial.
The hair follicle comprises several parts, the DP, dermal sheath, hair matrix, and outer root sheath7. The DP cells at the base of the hair follicle act as the signaling center of hair follicles controlling hair growth by secreting growth/regression signals from the adjacent hair matrix cells8. Several growth factors (e.g., vascular endothelial growth factor A (VEGFA), platelet-derived growth factor B (PDGFB), fibroblast growth factor (FGF), and insulin-like growth factor (IGF)) secreted by DP cells activate the proliferation and keratinization of hair matrix cells and extend the growth phase of hair follicles9, 10. Because the weakness of DP cell functions causes hair loss through reduced hair growth signals, understanding DP activation factors is crucial for developing novel approaches to prevent and treat hair loss.
Hair growth is strongly affected by hormones11. Dihydrotestosterone (DHT) is the most potent androgen that modulates hair growth. A double-blinded study showed that DHT levels are significantly higher in bald scalps than hair-containing scalps12. Finasteride, an oral drug that can decrease DHT levels, can delay the progression of alopecia and can be used as an effective drug for hair loss treatment13, 14. Estrogen and progesterone, sex hormones responsible for various female characteristics in the body, also regulate hair growth15. The levels of these hormones increase during pregnancy and cause additional hair growth in the head and body16, 17. A recent study has shown that cortisol, a stress-induced hormone, can cause hair loss18, 19. The stress hormone produced in the cortex of the adrenal gland inhibits hair regrowth by inhibiting Gas6 expression in DP18.
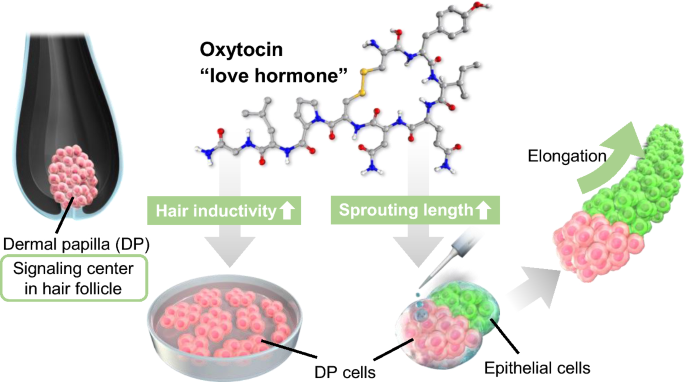
Oxytocin (OXT) is a pregnancy-related hormone secreted during childbirth and lactation. It is sometimes referred to as the “love hormone” because levels of OXT increase during hugging, massaging, music therapy, and interaction with pet dogs20,21,22. Recent studies have suggested that OXT is an anti-stress hormone, and clinical trials have shown that oxytocin provides therapeutic benefits for patients with stress-related disorders23. The positive effects of OXT on several organs, including the digestive, muscular, and reproductive systems, have been reported in recent years24, 25, but the understanding of the relationship between hair follicles and OXT remains unclear. Herein, we examined the effects of OXT on the hair growth ability of DP cells (Fig. 1). Cultured DP cells were treated with OXT and examined for cell proliferation and DP signature marker gene expression. Finally, the hair growth-promoting ability of OXT was investigated using an in vitro hair growth model. The findings of this study may help in the development of new treatment strategies for hair loss.

Scheme outlining the identification of oxytocin (OXT) effects on dermal papilla (DP) cells. DP cells and hair follicloids were supplemented with oxytocin for activation and hair growth promotion.
Results
Effects of OXT on DP cell function
OXT signal transduction begins after OXT binds to the OXT receptor (OXTR). We first confirmed the existence of OXTR for acceptance of OXT signals in DP cells. Immunocytochemical staining and Western blotting revealed that OXTR was expressed in DP cells (Fig. 2, Supplementary Figs. 1, 2). Several other cell sources, including microglia, islet cells, and cardiomyocyte, have OXTR and respond to OXT at 0–10 µM concentration26,27,28,29. Therefore, OXT treatment at different concentrations (0, 0.1, 1, and 10 µM) was administered to DP cells in 2D culture for 6 days. No significant difference in cell proliferation rate with OXT concentration was observed for 6 days (Fig. 3a). Known markers of DP signature, including versican (VCAN), alkaline phosphatase (ALP), lymphoid enhancer-binding factor 1 (LEF1), Wnt family member 5A (WNT5A), bone morphogenetic protein 4 (BMP4), and noggin (NOG), are generally correlated hair induction and growth capacity30,31,32,33. These gene expressions were gradually increased with OXT supplementation in a concentration-dependent manner (Fig. 3b). Among them, the levels of VCAN, ALP, and NOG were significantly upregulated by 10 µM OXT treatments (Fig. 3b, Supplementary Fig. 3). The VCAN, ALP, and NOG protein productions were also increased by 10 µM OXT treatments (Fig. 4, Supplementary Fig. 4).

Oxytocin receptor expression in dermal papilla (DP) cells. Cultured DP cells were stained with the anti-oxytocin receptor (OXTR); OXTR (green), nuclei (blue), and actin filaments (red) were visualized using confocal microscopy.

Proliferation and gene expression analysis of dermal papilla (DP) cells cultured in 2D. (a) Number of expanded cells at 3 and 6 days of culture. (b) Expression of DP signature marker genes. GAPDH was used as a reference gene to normalize expression. Error bars represent the standard error of the mean calculated from three experiments for each condition. Using Tukey’s test, the numerical variables were statistically evaluated; * indicates p < 0.05.

Western blotting analysis of dermal papilla (DP) cells cultured in 2D. Protein expression of VCAN, NOG, and ALP. Proteins in DP cells treated with 0 μM and 10 μM oxytocin (OXT) were analyzed by Western blotting. The intensity of chemiluminescence was normalized with GAPDH. The expression levels are shown as fold changes of the respective values without OXT.
We further investigated the key signaling pathways activated by OXT stimulation. DP cells were treated with/without 10 µM OXT for 6 days, and the gene expressions were investigated through comprehensive RNA-seq analysis. RNA-seq results revealed 18,929 DEGs between 0 and 10 µM OXT, of which 8480 genes were upregulated in 10 µM OXT-treated DP. The top 10 enriched pathways represented by these upregulated genes included the cytokine–cytokine receptor interaction and OXT signaling pathway (Fig. 5a). In addition, genes associated with hair growth-promoting factors, including VEGFA, FGF7, and BMP2, were also upregulated by 10 µM OXT treatment (Fig. 5b). These results suggest that mechanisms associated with DP cell activations include cytokine and growth factor secretion through the activation of OXT signaling pathway. Minoxidil, a commercially available hair growth reagent, improves DP cell function to increase the production of growth factors such as VEGF and FGF734, 35. Since OXT has a similar effect, we hypothesized that OXT might be a candidate for a hair growth reagent and investigated the hair growth ability of OXT using our in vitro hair follicle organoid models36.

Gene chip analysis and enriched signaling pathway inhibition. (a) Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis of upregulated differentially expressed genes (DEGs) showing the top 10 enriched pathways. (b) Heat map DEGs between DP cells treated with 0 μM and 10 μM oxytocin (OXT).
Hair growth assay using an in vitro hair growth model
We recently developed an in vitro hair follicle organoid model (termed the hair follicloid) to identify hair growth-promoting factors36. In our previous study, hair follicloids were prepared by culturing human DP and epithelial cells in a medium supplemented with a low concentration of Matrigel. Matrigel significantly enhanced the self-organization capabilities of epithelial and mesenchymal cells, resulting in spherical aggregation and subsequent hair peg-like sprouting. Peg-like hair sprouting is composed of hair cortex cell marker AE13 positive cells, but the structure is immature compared with native hair follicles. However, the hair follicloid was sufficient to elongate the peg-like hair sprouting in response to minoxidil. In the present study, we used hair follicloids to examine the effect of OXT on hair growth. Human DP and epithelial cells were suspended in a medium supplemented with 2 v/v% Matrigel and cultured in 96 well spheroid formation plates to prepare hair follicloids. Hair follicloids were treated with 0 and 10 µM OXT after day 4, and hair peg-like sprouting was measured from day 4 to 10 (Fig. 6a). Hair peg-like sprouting was elongated both in 0 and 10 µM OXT-treated hair follicloids. The sprouting length was significantly longer in the 10 µM OXT-treated group than in the untreated control group on days 8 and 10 (Fig. 6b,c). Next, we examined the expression of DP-produced growth factors, including VEGFA and PDGFB. VEGFA and PDGFB were significantly upregulated in the presence of OXT (Fig. 6d), suggesting that the elongation of hair peg-like sprouts was promoted by the production of growth factors. These results indicate that OXT has the potential to be a hair growth reagent, although further studies are needed to determine their effects under in vivo environments of hair follicles.

Hair growth testing using hair follicloids. (a) Procedures for testing the effects of oxytocin (OXT). (b) Microscope images of hair follicloids cultured with/without OXT for 10 days. Hair follicloids were permeabilized and observed using a stereomicroscope. (c) The length of sprouting structures with/without OXT. The graph shows the length ratio on days 6, 8, and 10 compared to day 4. (d) Relative expression of hair growth-associated genes. GAPDH was used as a reference gene to normalize expression. Error bars represent the standard error of the mean calculated from three experiments for each condition. Numerical variables were statistically evaluated using Student’s t-test; * indicates p < 0.05.
Discussion
A recent study has shown that OXT accumulates in hair shafts and can be evaluated as a biomarker of stress37; however, to the best of our knowledge, there are no studies on the effects of OXT on cells in hair follicles. We found that OXT stimulates DP cells to promote the secretion of growth factors. This finding inspired us to apply OXT therapy to the hair loss treatment, and the hair growth-promoting effects of OXT were confirmed using our in vitro drug screening model. This study revealed the roles of OXT in hair follicles in in vitro, but it would be crucial to understand whether OXT affects hair follicles in more complex in vivo environments for appreciation as a hair loss treatment drug. OXT has been shown to act on multiple cell types in addition to hair follicles24, 25, and its use as a treatment for alopecia requires consideration of its side effects on multiple organs. The future study will investigate the understanding of hair growth and side effects using an alopecia model mouse. Also, the present study used DP cells derived from healthy donors, and further investigations must be conducted using cells from patients with alopecia. In addition, understanding age- and sex-dependent efficacy is necessary because OXT production differs with age and gender. OXT concentration and treatment time should be more precisely optimized through these experiments. By advancing these studies, we would like to verify whether OXT can be a new drug for the treatment of alopecia.
The transcriptome analysis of the DP cells revealed that OXT treatment promoted OXT signal transduction and cytokine/growth factor secretion. The DP-secreted factors promoted the growth of hair peg-like sprouting in hair follicloids. However, there are many possible mechanisms by which OXT promotes hair growth. It should be noted that epithelial cells in hair follicloids also have OXTR (Supplementary Fig. 5). Further investigation is needed to understand whether OXT acts directly on epithelial cells to stimulate cell proliferation and growth of hair peg-like sprouting.
Although we investigated the effect of OXT as a hair growth-promoting drug, it may also be used to restore DP cell function in hair regeneration medicine. When DP cells are transplanted with epithelial cells into the skin, DP cells can induce de novo hair follicle regeneration in the recipient’s skin38. However, the hair regeneration ability of DP cells is gradually lost during expansion culture39. We previously developed approaches to restore the hair regeneration ability of DP cells using electrical stimulation and gel bead culturing40,41,42. Combining these methods with OXT may further improve the hair regeneration efficiency of expanded DP cells, which will be examined in future studies.
In conclusion, we showed that OXT activated the DP cells to promote growth factor secretion for hair growth. These findings encourage further investigation for clinical applications of OXT therapy in patients suffering from hair loss.
Methods
Preparation of human DP and epithelial cells
Adult human DP cells were obtained from PromoCell (Heidelberg, Germany), passaged up to passage four with R-STEM in hMSC high-growth medium (EM1; Roto, Japan), and used for 2D culture. Adult human follicular keratinocytes (epithelial cells) were obtained from ScienCell Research Laboratories (Carlsbad, CA, USA). DP cells at passage four and epithelial cells at passage one were used for organoid culture. Incubator gas tension was maintained at 21% O2 and 5% CO2 at 37 °C.
OXT treatment of DP cells
DP cells (2 × 104 cells) were suspended in 0.5 mL EM1 medium supplemented with 0, 0.1, 1, or 10 μM OXT (Peptide Institute Inc., Japan) and seeded into the wells of a 24-well cell culture plate (Corning Inc., Corning, NY, USA). The culture medium was replaced with a fresh medium every 3 days. The cells were counted using a cell counter (Chemometec, Denmark) after 3 min of trypsin–EDTA treatment. Gene expression in DP cells was assessed using real-time reverse transcription-polymerase chain reaction (RT-PCR) after 6 days of culture.
OXT treatment of hair follicloids
To investigate the effects of OXT on hair growth, DP cells (5 × 103 cells) and epithelial cells (5 × 103 cells) were suspended in 0.2 mL advanced Dulbecco’s Modified Eagle Medium/Nutrient Mixture F-12 (DMEM/F-12; Thermo Fisher Scientific) containing 2% (v/v) Matrigel (Corning Inc.) and seeded into the wells of a non-cell-adhesive round-bottom 96-well plate (Primesurface® 96U plate; Sumitomo Bakelite Co., Ltd., Japan). The DMEM/F-12 medium was supplemented with 10 μM OXT from days 4–10 after seeding. Then, 0.1 mL of the spent medium was replaced with the same fresh medium every 2 days. Hair sprout lengths were observed using an all-in-one fluorescence microscope (BZ-X810, Keyence).
Immunocytochemical staining
DP cells were cultured with EM1 for 3 days for immunocytochemical staining and fixed with 4% (v/v) paraformaldehyde (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan) for 10 min. The samples were washed three times with phosphate-buffered saline (PBS) and blocked in blocking solution [PBS containing 3% (v/v) normal goat serum (Abcam Cambridge, UK) and 0.3% (v/v) Triton-X (Sigma Aldrich)] for 1 h at 25 °C. Next, the cells were incubated for 1 h with anti-OXTR (1:200 dilution, 23045-1-AP, Proteintech, Rosemont, IL, USA) at 25 °C. The samples were washed three times with blocking solution and incubated with the corresponding Goat Anti-Rabbit IgG H&L (Alexa Fluor® 488) antibody (1:500 dilution, ab150077, Abcam) in the blocking solution for 1 h at 25 °C and lastly with rhodamine-phalloidin (ab235138, Abcam) and 4′,6-diamidino-2-phenylindole (DAPI; ab228549, Abcam) in PBS for 30 min. A confocal microscope (LSM 700; Carl Zeiss, Germany) was used for fluorescence imaging.
Gene expression analysis
Total RNA was extracted from the samples using RNeasy Mini Kit (Qiagen, Hilden, Germany) and used for complementary DNA synthesis using the ReverTra Ace® RT-qPCR Kit (Toyobo, Osaka, Japan) according to the manufacturer’s instructions. Subsequent qRT-PCRs were performed using the StepOne Plus RT-PCR system (Applied Biosystems, Waltham, MA, USA) with SYBR® Premix Ex Taq™ II (Takara Bio, Kusatsu, Japan) and primers for amplifying human ALP, VCAN, LEF1, WNT5A, BMP4, NOG, VEGFA, PDGFB, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Table 1). All primers used in this study are listed in Table S1. All gene expression levels were normalized to that of GAPDH. The 2−∆∆Ct method was used to determine relative gene expression levels, and they were presented as the mean ± standard error of three independent experiments. Statistical evaluation of numerical variables was conducted using Tukey’s or Student’s t-test, where a p-value of < 0.05 indicated statistical significance.
Western blotting
Cell lysates are prepared in RIPA lysis buffer (EzRIOA Lysis kit; ATTO, Tokyo, Japan) as described previously43. Briefly, cells were washed with ice-cold PBS and lysed with RIPA lysis buffer. After incubation at 4 °C for 30 min, the cell lysate was centrifuged at 12,000 rpm for 10 min. The supernatant was collected and sequentially mixed with 2 × sodium dodecyl sulfate (SDS) sample buffer. The proteins were separated by electrophoresis on SDS-PAGE gels (Bio-Rad, Hercules, CA, USA) and transferred onto Immobilon-P membranes (Merck KGaA, Darmstadt, Germany). After blocking step with 3% BSA in Tris-buffered saline containing 0.1% Tween-20 (TBST) for 1 h at room temperature, the blots were incubated with primary antibodies (OXTR, 1:1000 dilution, 23045-1-AP, Proteintech; Versican, 1:1000 dilution, PA1-1748A, Thermo Fisher Scientific; ALP, 1:500 dilution, ab229126, Abcam; Noggin, 1:1000 dilution, ab16054, Abcam; GAPDH, 1:2000 dilution, 5174, Cell Signaling Technology) at 4 °C overnight. The membranes were washed with TBST solution three times, then incubated with a horseradish peroxidase-conjugated secondary antibody (1:2000 dilution, Cell Signaling Technology) at room temperature for 1 h. Protein bands on the membrane were visualized using ECL Prime (GE Healthcare, Buckinghamshire, UK) and an Amersham Imager 600 RGB (Cytiva, Tokyo, Japan). The relative protein levels were evaluated using the GAPDH expression level as a reference.
RNA-seq analysis
Total RNA was extracted using RNeasy Mini Kit (Qiagen) from DP cells with/without 10 μM OXT treatment for 6 days. RNA-seq analysis was performed by Takara Bio. The significantly upregulated genes in DP cells subjected to OXT treatment were used for the Kyoto Encyclopedia of Genes and Genomes pathway analysis with the Database for Annotation, Visualization, and Integrated Discovery (http://david.abcc.ncifcrf.gov/)44,45,46.
Statistical analysis
Statistical analyses of gene expression levels and the length of hair sprouts were conducted using Tukey’s test or Student’s t-test, and the results were considered statistically significant at p < 0.05. All data are presented as mean ± standard error.
Data availability
The datasets generated and analyzed during the current study are available in the NCBI repository, GSE233904.
References
-
Phillips, T. G., Slomiany, W. P. & Allison, R. Hair loss: Common causes and treatment. Am. Fam. Phys. 96, 371–378 (2017).
-
Lin, R. L., Garibyan, L., Kimball, A. B. & Drake, L. A. Systemic causes of hair loss. Ann. Med. 48, 393–402. https://doi.org/10.1080/07853890.2016.1180426 (2016).
Google Scholar
-
Nestor, M. S., Ablon, G., Gade, A., Han, H. & Fischer, D. L. Treatment options for androgenetic alopecia: Efficacy, side effects, compliance, financial considerations, and ethics. J. Cosmet. Dermatol. 20, 3759–3781. https://doi.org/10.1111/jocd.14537 (2021).
Google Scholar
-
Shiell, R. C. A review of modern surgical hair restoration techniques. J. Cutan. Aesthet. Surg. 1, 12–16. https://doi.org/10.4103/0974-2077.41150 (2008).
Google Scholar
-
Sung, J. H. Effective and economical cell therapy for hair regeneration. Biomed. Pharmacother. 157, 113988. https://doi.org/10.1016/j.biopha.2022.113988 (2023).
Google Scholar
-
Santos, Z., Avci, P. & Hamblin, M. R. Drug discovery for alopecia: Gone today, hair tomorrow. Expert Opin. Drug Discov. 10, 269–292. https://doi.org/10.1517/17460441.2015.1009892 (2015).
Google Scholar
-
Schneider, M. R., Schmidt-Ullrich, R. & Paus, R. The hair follicle as a dynamic miniorgan. Curr. Biol. 19, R132-142. https://doi.org/10.1016/j.cub.2008.12.005 (2009).
Google Scholar
-
Millar, S. E. Molecular mechanisms regulating hair follicle development. J. Investig. Dermatol. 118, 216–225. https://doi.org/10.1046/j.0022-202x.2001.01670.x (2002).
Google Scholar
-
Wall, D., Meah, N., Fagan, N., York, K. & Sinclair, R. Advances in hair growth. Fac. Rev. 11, 1. https://doi.org/10.12703/r/11-1 (2022).
Google Scholar
-
Shome, D., Kapoor, R., Surana, M., Vadera, S. & Shah, R. Efficacy of QR678 Neo((R)) hair growth factor formulation for the treatment of hair loss in Covid-19-induced persistent Telogen Effluvium-A prospective, clinical, single-blind study. J. Cosmet. Dermatol. 21, 16–23. https://doi.org/10.1111/jocd.14626 (2022).
Google Scholar
-
Grymowicz, M. et al. Hormonal effects on hair follicles. Int. J. Mol. Sci. 21, 5342. https://doi.org/10.3390/ijms21155342 (2020).
Google Scholar
-
Dallob, A. L. et al. The effect of finasteride, a 5 alpha-reductase inhibitor, on scalp skin testosterone and dihydrotestosterone concentrations in patients with male pattern baldness. J. Clin. Endocrinol. Metab. 79, 703–706. https://doi.org/10.1210/jcem.79.3.8077349 (1994).
Google Scholar
-
Inadomi, T. Efficacy of finasteride for treating patients with androgenetic alopecia who are pileous in other areas: A pilot study in Japan. Indian J. Dermatol. 59, 163–165. https://doi.org/10.4103/0019-5154.127677 (2014).
Google Scholar
-
McClellan, K. J. & Markham, A. Finasteride: A review of its use in male pattern hair loss. Drugs 57, 111–126. https://doi.org/10.2165/00003495-199957010-00014 (1999).
Google Scholar
-
Randall, V. A. Hormonal regulation of hair follicles exhibits a biological paradox. Semin. Cell Dev. Biol. 18, 274–285. https://doi.org/10.1016/j.semcdb.2007.02.004 (2007).
Google Scholar
-
Gizlenti, S. & Ekmekci, T. R. The changes in the hair cycle during gestation and the post-partum period. J. Eur. Acad. Dermatol. Venereol. 28, 878–881. https://doi.org/10.1111/jdv.12188 (2014).
Google Scholar
-
Conrad, F., Ohnemus, U., Bodo, E., Bettermann, A. & Paus, R. Estrogens and human scalp hair growth-still more questions than answers. J. Investig. Dermatol. 122, 840–842. https://doi.org/10.1111/j.0022-202X.2004.22344.x (2004).
Google Scholar
-
Choi, S. et al. Corticosterone inhibits GAS6 to govern hair follicle stem-cell quiescence. Nature 592, 428–432. https://doi.org/10.1038/s41586-021-03417-2 (2021).
Google Scholar
-
Thom, E. Stress and the hair growth cycle: Cortisol-induced hair growth disruption. J. Drugs Dermatol. 15, 1001–1004 (2016).
Google Scholar
-
Shen, H. Neuroscience: The hard science of oxytocin. Nature 522, 410–412. https://doi.org/10.1038/522410a (2015).
Google Scholar
-
Florea, T. et al. Oxytocin: Narrative expert review of current perspectives on the relationship with other neurotransmitters and the impact on the main psychiatric disorders. Medicina (Kaunas) 58, 923. https://doi.org/10.3390/medicina58070923 (2022).
Google Scholar
-
Marshall-Pescini, S. et al. The role of oxytocin in the dog-owner relationship. Animals (Basel) 9, 792. https://doi.org/10.3390/ani9100792 (2019).
Google Scholar
-
Takayanagi, Y. & Onaka, T. Roles of oxytocin in stress responses, allostasis and resilience. Int. J. Mol. Sci. 23, 150. https://doi.org/10.3390/ijms23010150 (2021).
Google Scholar
-
Kerem, L. & Lawson, E. A. The effects of oxytocin on appetite regulation, food intake and metabolism in humans. Int. J. Mol. Sci. 22, 7737. https://doi.org/10.3390/ijms22147737 (2021).
Google Scholar
-
Liu, N., Yang, H., Han, L. & Ma, M. Oxytocin in women’s health and disease. Front. Endocrinol. (Lausanne) 13, 786271. https://doi.org/10.3389/fendo.2022.786271 (2022).
Google Scholar
-
Wasserman, A. H. et al. Oxytocin promotes epicardial cell activation and heart regeneration after cardiac injury. Front. Cell Dev. Biol. 10, 985298. https://doi.org/10.3389/fcell.2022.985298 (2022).
Google Scholar
-
Hsieh, F. F. et al. Maternal oxytocin administration modulates gene expression in the brains of perinatal mice. J. Perinat. Med. 50, 207–218. https://doi.org/10.1515/jpm-2020-0525 (2022).
Google Scholar
-
Gu, P., Lin, Y., Wan, Q., Su, D. & Shu, Q. Oxytocin signal contributes to the adaptative growth of islets during gestation. Endocr. Connect. 10, 694–706. https://doi.org/10.1530/EC-21-0043 (2021).
Google Scholar
-
Inoue, T. et al. Oxytocin suppresses inflammatory responses induced by lipopolysaccharide through inhibition of the eIF-2-ATF4 pathway in mouse microglia. Cells 8, 527. https://doi.org/10.3390/cells8060527 (2019).
Google Scholar
-
Veraitch, O. et al. Induction of hair follicle dermal papilla cell properties in human induced pluripotent stem cell-derived multipotent LNGFR(+)THY-1(+) mesenchymal cells. Sci. Rep. 7, 42777. https://doi.org/10.1038/srep42777 (2017).
Google Scholar
-
Yoshida, Y., Soma, T., Matsuzaki, T. & Kishimoto, J. Wnt activator CHIR99021-stimulated human dermal papilla spheroids contribute to hair follicle formation and production of reconstituted follicle-enriched human skin. Biochem. Biophys. Res. Commun. 516, 599–605. https://doi.org/10.1016/j.bbrc.2019.06.038 (2019).
Google Scholar
-
Kishimoto, J. et al. Selective activation of the versican promoter by epithelial–mesenchymal interactions during hair follicle development. Proc. Natl. Acad. Sci. U. S. A. 96, 7336–7341. https://doi.org/10.1073/pnas.96.13.7336 (1999).
Google Scholar
-
Zhou, P., Byrne, C., Jacobs, J. & Fuchs, E. Lymphoid enhancer factor 1 directs hair follicle patterning and epithelial cell fate. Genes Dev. 9, 700–713. https://doi.org/10.1101/gad.9.6.700 (1995).
Google Scholar
-
Lee, C. Y. et al. Hair growth is promoted by BeauTop via expression of EGF and FGF-7. Mol. Med. Rep. 17, 8047–8052. https://doi.org/10.3892/mmr.2018.8917 (2018).
Google Scholar
-
Lachgar, S., Charveron, M., Gall, Y. & Bonafe, J. L. Minoxidil upregulates the expression of vascular endothelial growth factor in human hair dermal papilla cells. Br. J. Dermatol. 138, 407–411. https://doi.org/10.1046/j.1365-2133.1998.02115.x (1998).
Google Scholar
-
Kageyama, T., Miyata, H., Seo, J., Nanmo, A. & Fukuda, J. In vitro hair follicle growth model for drug testing. Sci. Rep. 13, 4847. https://doi.org/10.1038/s41598-023-31842-y (2023).
Google Scholar
-
Lopez-Arjona, M. et al. A procedure for oxytocin measurement in hair of pig: Analytical validation and a pilot application. Biology (Basel) 10, 527. https://doi.org/10.3390/biology10060527 (2021).
Google Scholar
-
Jahoda, C. A., Horne, K. A. & Oliver, R. F. Induction of hair growth by implantation of cultured dermal papilla cells. Nature 311, 560–562. https://doi.org/10.1038/311560a0 (1984).
Google Scholar
-
Higgins, C. A., Chen, J. C., Cerise, J. E., Jahoda, C. A. & Christiano, A. M. Microenvironmental reprogramming by three-dimensional culture enables dermal papilla cells to induce de novo human hair-follicle growth. Proc. Natl. Acad. Sci. U. S. A. 110, 19679–19688. https://doi.org/10.1073/pnas.1309970110 (2013).
Google Scholar
-
Kageyama, T., Yan, L., Shimizu, A., Maruo, S. & Fukuda, J. Preparation of hair beads and hair follicle germs for regenerative medicine. Biomaterials 212, 55–63. https://doi.org/10.1016/j.biomaterials.2019.05.003 (2019).
Google Scholar
-
Yan, L. et al. Electrical stimulation to human dermal papilla cells for hair regenerative medicine. J. Biosci. Bioeng. 133, 281–290. https://doi.org/10.1016/j.jbiosc.2021.12.003 (2022).
Google Scholar
-
Yamane, M. et al. Effects of the PI3K/Akt signaling pathway on the hair inductivity of human dermal papilla cells in hair beads. J. Biosci. Bioeng. 134, 55–61. https://doi.org/10.1016/j.jbiosc.2022.03.010 (2022).
Google Scholar
-
Seo, J. et al. Hypoxia inducible factor-1alpha promotes trichogenic gene expression in human dermal papilla cells. Sci. Rep. 13, 1478. https://doi.org/10.1038/s41598-023-28837-0 (2023).
Google Scholar
-
Kanehisa, M. & Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28, 27–30. https://doi.org/10.1093/nar/28.1.27 (2000).
Google Scholar
-
Kanehisa, M. Toward understanding the origin and evolution of cellular organisms. Protein Sci. 28, 1947–1951. https://doi.org/10.1002/pro.3715 (2019).
Google Scholar
-
Kanehisa, M., Furumichi, M., Sato, Y., Kawashima, M. & Ishiguro-Watanabe, M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 51, D587–D592. https://doi.org/10.1093/nar/gkac963 (2023).
Google Scholar
Acknowledgements
This work was supported by the Kanagawa Institute of Industrial Science and Technology (KISTEC) and Grants-in-Aid for Scientific Research (KAKENHI; Grant number 20H02535, 23K13614, 23H01771).
Author information
Authors and Affiliations
Contributions
T.K. and J.F. designed the experiments. T.K. conducted the experiments. T.K., S.J., L.Y., and J.F. prepared the manuscript. J.F. contributed to the manuscript submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Information.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
Reprints and Permissions
About this article
Cite this article
Kageyama, T., Seo, J., Yan, L. et al. Effects of oxytocin on the hair growth ability of dermal papilla cells.
Sci Rep 13, 15587 (2023). https://doi.org/10.1038/s41598-023-40521-x
-
Received: 26 May 2023
-
Accepted: 11 August 2023
-
Published: 20 October 2023
-
DOI: https://doi.org/10.1038/s41598-023-40521-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.