Abstract
Pesticides are widely used in global agriculture to achieve high productivity levels. Among them, fungicides are specifically designed to inhibit fungal growth in crops and seeds. However, their application often results in environmental contamination, as these chemicals can persistently be detected in surface waters. This poses a potential threat to non-target organisms, including humans, that inhabit the affected ecosystems. In toxicologic research, the zebrafish (Danio rerio) is the most commonly used fish species to assess the potential effects of fungicide exposure, and numerous and sometimes conflicting findings have been reported. To address this, we conducted a systematic review and meta-analysis focusing on the neurobehavioral effects of fungicides in zebrafish. Our search encompassed three databases (PubMed, Scopus, and Web of Science), and the screening process followed predefined inclusion/exclusion criteria. We extracted qualitative and quantitative data, as well as assessed reporting quality, from 60 included studies. Meta-analyses were performed for the outcomes of distance traveled in larvae and adults and spontaneous movements in embryos. The results revealed a significant overall effect of fungicide exposure on distance, with a lower distance traveled in the exposed versus control group. No significant effect was observed for spontaneous movements. The overall heterogeneity was high for distance and moderate for spontaneous movements. The poor reporting practices in the field hindered a critical evaluation of the studies. Nevertheless, a sensitivity analysis did not identify any studies skewing the meta-analyses. This review underscores the necessity for better-designed and reported experiments in this field.
Introduction
Chemical pesticides are synthetical active ingredients used to control pests that may threaten the productivity of crops1. To yield high productivity levels, modern agriculture employs large amounts of pesticides2. In 2020, the global consumption of these products reached almost 3 million tonnes3. The substantial quantity and the method by which they are applied results in environmental contamination of the soil, surface waters, and food4,5,6. Data shows that less than 0.1% of the pesticide hits the intended target species, leaving the remaining residual impacting the environment and public health7. Its presence in superficial waters generates risk to the non-target organisms by decreasing biodiversity and the population of primary food chain producers and reducing the prey for the aquatic organisms8,9,10. Moreover, the dissemination of pesticides in the environment represents a risk to humans, whereas their presence in the water supply leads to potential consumption11,12,13,14.
According to the target organism, these substances can be classified as herbicides, insecticides, rodenticides, and fungicides15, being the fungicides one of the most used chemicals16. Their application aims to kill and/or inhibit fungal growth in agriculture, both in seeds and crops17.
Due to the need to understand the effects of exposure to these products, the scientific literature presents several studies with animals in this area18. The model organism zebrafish (Danio rerio, Hamilton 1822) is widely used in toxicology, mostly because of its high fecundity, fast development, transparency of the embryo, and high homology of organs and genetics concerning humans19,20,21. In addition, the zebrafish is an aquatic animal that dwells in potentially contaminated ecosystems, representing the eventual consequences of exposure to other cohabitant species22. It has been reported that exposure to fungicides in zebrafish causes behavioral, neurochemical, developmental, metabolic, hormonal, hepatotoxic, cardiotoxic, enzymatic, morphological, and molecular alterations23,24,25,26,27,28.
From 2012 to 2019, more than 100 articles were published investigating the effects of fungicides in zebrafish, which represents the second most investigated type of pesticide in this organism29. However, there is a high methodological heterogeneity between the studies. The interventions, developmental stages, and outcomes addressed are extremely variable between studies. Regarding the intervention, plenty of compounds used as fungicides exhibit distinct mechanisms of action30 and can be administered over a wide range of durations through multiple routes of administration. As for the developmental stage, in vivo exposure can be performed in embryos, larvae, or adults; the outcomes are distinctly selected according to the research question and the capabilities of the research group (neurotoxicity, hepatotoxicity, cardiotoxicity, among others)31.
Many studies were published on the toxic effects of fungicides on neurobehavioral parameters in zebrafish22,32. However, no secondary studies systematically synthesize these results to obtain an understanding supported by published evidence to optimize the planning of new research. An accurate description of these preclinical data and a meta-analysis can help avoid redundant studies and the consequent use of animals. Furthermore, considering the reproducibility issues raised for the zebrafish research field33,34, it is essential to identify possible sources of bias and conflicting results, including assessing the quality of available publications. This systematic review and meta-analysis of literature aimed at synthesizing the data from neurobehavioral effects of fungicide exposure in zebrafish, also analyzing reporting quality and publication bias.
Methods
Before screening studies and data extraction, a protocol guiding this review was registered in Open Science Framework, and preregistration is available at https://osf.io/f2d3835. The reporting of this study follows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines36.
Search strategy
The studies were identified through a search in the literature using three different databases: PubMed, Scopus, and Web of Science. The search strategies were designed to adapt to each database characteristics. The terms were combined for the intervention (fungicide exposure) and the population of interest (zebrafish), aiming to conduct a comprehensive search, including all the available articles that fulfilled the inclusion criteria. The complete query for each database can be found at https://osf.io/5ae9q35. The strategy did not apply any search filter, language restriction, or limit of year. The search was performed on the 1st of December, 2021, and the articles were imported to Rayyan software37 to identify and remove the duplicates.
Study screening
Initially, the retrieved studies from the three databases were analyzed to filter and exclude duplicates (performed by CGR). The remaining articles were pre-selected based on their title and abstract. If a reason to exclude the record was not found, at this stage, it was carried forward to the full-text screening stage. In both stages (title/abstract and full-text), two independent reviewers (CGR and LMB, RC or SZB) examined each study. Disagreements between the decisions of the reviewers were resolved by a third reviewer (QKZ, AP, or APH).
Experimental studies evaluating the effects of exposure to fungicides in zebrafish on the following parameters were included: motor function, sensory function, learning and memory, social behavior, sexual behavior, eating behavior, anxiety-like or fear-related behaviors, behaviors related to the reward system, and behaviors related to circadian rhythms. The parameters were included only if they were linked to the central nervous system. The identity of the compound as a fungicide was consulted in the Pesticide Properties Database38.
In the first phase (screening of title/abstract), papers were excluded according to the following criteria:
-
1.
Type of study design: reviews, comments, abstracts published in conference proceedings, corrections, editorials;
-
2.
Type of population: in vitro investigations or studies with species other than zebrafish;
-
3.
Type of intervention: biological and commercial formulations or mixtures of fungicides, non-interventional studies;
In the next phase (full-text screening), the following criteria were added, and the articles were excluded based on the above items plus:
-
4.
Comparison: when there is no proper control group (same organism, same procedure, except for fungicide exposure);
-
5.
Outcome measures: if there is no assessment of any previously cited neurobehavioral outcome.
More information about this section is available at https://osf.io/wmsvg.
Data extraction
Two independent investigators (CGR and LMB, RC or SZB) performed the data extraction, and a discussion between the two reviewers resolved disagreements. The information and values of interest were directly extracted from the text and tables. When not possible, WebPlotDigitizer software (v4.5, Rohatgi, A., Pacifica, CA, USA, https://automeris.io/WebPlotDigitizer) was used to determine the values from the graphs manually. The following data were extracted: (1) study identification: study title, digital object identifier (DOI), first author, last author, year of publication, and last author affiliation; (2) model animal specifications: strain, sex, the developmental stage during exposure, age during exposure, the developmental stage during the test, age during the test; (3) fungicide exposure characteristics: fungicide, administration route and type (i.e., static, semi-static or flow through), frequency of renewal, frequency of exposure, duration of exposure, dose/concentration and the interval between exposure and test; (4) test properties: test nomenclature, category of measured variable (e.g., anxiety, locomotor, social) and the measured variable.
Regarding the authors of the studies, co-authorship networks were elaborated using VOSviewer software version 1.6.18 (https://www.vosviewer.com)39,40.
Data were collected for each variable according to the outcomes of interest, including the mean and the number of animals (n) for both the control and exposed groups. The standard deviation (SD) or standard error of the mean (SEM) was extracted for the reported mean value. If the SEM was reported, the SD was calculated by multiplying SEM by the square root of the sample size (SD = SEM ∗ √n).
In instances where the sample size was reported as a range, the lowest value was used. Whenever information was unclear or missing, attempts were made to contact the corresponding author of the study via email, with two separate attempts made at least two weeks apart.
Reporting quality
To assess the reporting quality of included studies, two independent reviewers (CGR and LMB, RC, or SZB) evaluated each paper based on41, which proposes criteria for transparent reporting. The observed topics were: (1) mention of any randomization process; (2) sample size estimation; (3) mention of inclusion/exclusion criteria; (4) mention of any process to ensure blinding during the experiments. A score of “yes” or “no” was given for each topic, meaning that it was or was not reported, respectively. The outcome measurements performed by any automated software were considered blinded. Reporting quality plots were created using robvis42.
A complete guide for assessing the reporting quality associated with each item in this review is available at https://osf.io/uy5v3.
Meta-analysis
To perform a meta-analysis, at least 5 studies with the same outcome were required a priori35. Whenever two or more experimental groups shared the same control, the sample size of the control group was divided by the number of comparisons and then rounded down. Further information about the basic aspects of our method can be found at43.
Effects sizes were determined with the standardized mean difference (SMD) using Hedge’s G method44. SMD was used because studies examined a common outcome while employing different measurement approaches, which makes it necessary to standardize the findings in a uniform scale to allow combination across studies. Briefly, SMD expresses the size of an intervention effect relative to the observed variability45,46. Analyses were conducted using R Project for Statistical Computing with packages meta47 (https://cran.r-project.org/package=meta) and ggplot248 following Hedge’s random effects model, given the anticipated heterogeneity between studies. Values for SMD were reported with 95% confidence intervals. Heterogeneity between studies was estimated using I249, τ2, and Cochran’s Q50 tests. Heterogeneity variance (τ2) was estimated using the restricted maximum likelihood estimator51,52. The confidence intervals around pooled effects were corrected using Knapp-Hartung adjustments53. Values of 25%, 50%, and 75% were considered as representing low, moderate, and high heterogeneity, respectively, for I2, and a p-value ≤ 0.1 was considered significant for Cochran’s Q. Prediction intervals were estimated and represent the range of effects expected for future studies45. Furthermore, a subgroup meta-analysis was performed to evaluate if the developmental stage of the animals was a potential source of heterogeneity. Studies were grouped into two categories: larval and adult. Subgroup analysis was only performed when there were at least five unique studies for each subgroup. A p-value ≤ 0.1 was considered significant for subgroup differences54.
We conducted an exploratory meta-analysis to investigate an association between the effect and the fungicide class by categorizing them based on their chemical structure. Even without reaching the minimum of 5 studies, we ran a meta-analysis with 4 articles investigating fungicides of the triazole and anilide groups.
A mixed-effects meta-regression analysis was conducted to explore the relationship between the effect sizes and fungicide concentration as a moderator variable. The random effects structure accounted for potential heterogeneity across studies55. Meta-regressions excluding studies based on the sensitivity analysis were also performed.
Publication bias was investigated by generating funnel plots and performing Duval and Tweedie’s trim and fill analysis56 and Egger’s regression test57. Analyses were only conducted when at least five studies were available within a given outcome for funnel plots and at least ten studies for the regression test. A p-value < 0.1 was considered significant for the regression test.
Sensitivity analysis
A sensitivity analysis was conducted to assess if any experimental or methodological difference between studies was biasing the main effect found in the meta-analysis. Analyses were performed following the “leave-one-out jackknife method”58. A minimum of three comparisons were required for each outcome to conduct a sensitivity analysis. Furthermore, we conducted complementary meta-analyzes excluding studies that, when omitted in the leave-one-out, had observations that changed the overall effect direction. We also ran meta-analyses excluding studies containing experiments with atypically high SMD, as seen in the forest plots59.
Results
Search results
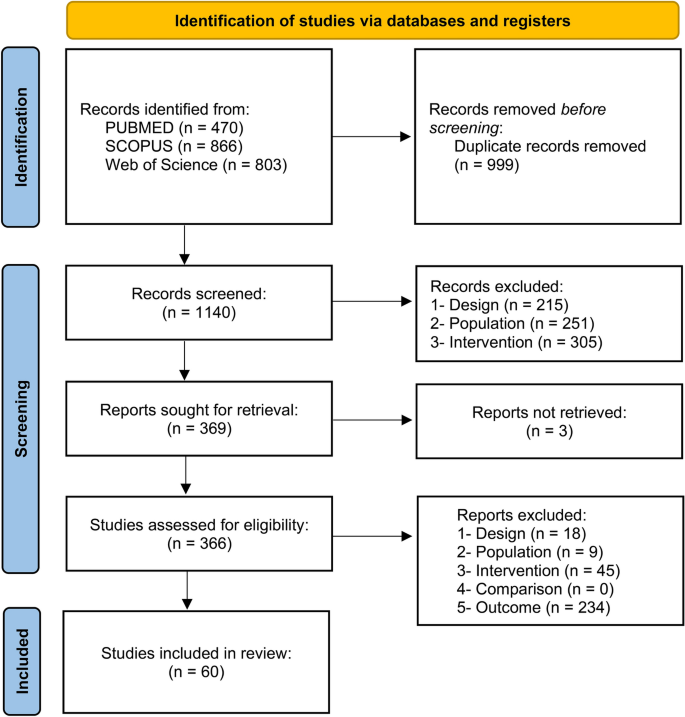
The search in the three databases retrieved a total of 2139 results. After removing duplicates, 1140 articles were screened for eligibility by analyzing the titles and abstracts. As a result of the first screening phase, 369 studies remained to be assessed based on their full-text. At this phase, 3 were not retrieved, and 60 fulfilled the criteria and were included in the review (Fig. 1). The main overall reasons for the exclusions were outcome (n = 234), population (n = 260), and intervention (n = 350). Concerning the quantitative synthesis, 8 studies were excluded because the minimum number of studies to perform a meta-analysis was not reached for the reported outcomes and 10 because of missing information. There were 18 experiments measuring distance using luminous transitions (dark/light) in larvae that were not included due to the variations between the protocols60, which makes the comparison infeasible. This resulted in 24 studies included in the quantitative synthesis. Detailed reasons for excluding studies from the meta-analysis are available at https://osf.io/qpcew.

Flowchart diagram of the collection of studies and selection process.
Study characteristics
A qualitative description of the studies is provided in Table 1. The identification of the studies was attributed according to the table available at https://osf.io/85d2p. A total of 43 different fungicides were addressed in the articles included in this review. Studies with the fungicides difenoconazole (n = 5, 8.3%), boscalid (n = 4, 6.6%), and pyraclostrobin (n = 4, 6.6%) were the most frequent.
All the studies used immersion as the exposure method, whereas exposure durations ranged from 11 min to 217 days. The most recurrent duration of exposure among the publications was 24 h (n = 21, 35%), followed by 96 h (n = 12, 20%). It is important to emphasize that 24 h is usually employed to verify the outcome of spontaneous movements, while 96 h is recommended by the Organization for Economic Co-operation and Development (OECD) to assess acute fish toxicity in protocols 203 (adults) and 236 (embryos)61,62. Regarding the developmental stage during the exposure, the embryonic was the most common (n = 52, 86.7%). Subsequently, the larval stage was observed in 7 studies (11.6%) and the adult stage in 8 (13.3%). Some articles used more than one stage for the exposure.
The outcome assessment was mostly performed in larvae (n = 41, 68.33%) and embryos (n = 25, 41.7%). Some studies assessed the outcomes in more than one developmental stage.
The sex of the adult animals was mainly reported as an equal proportion of male and female (F:M), except for one in which it was not reported (unclear).
Regarding the authors included in this review, co-authorship network analysis identified 24 clusters of researchers investigating the neurobehavioral effects of fungicides globally (Fig. S1). An interactive version of the co-authorship network is available at https://tinyurl.com/239thp6t.
Reporting quality
The summary plot of the reporting quality evaluation is shown in Fig. 2. Randomization process was not cited in 17 studies (28.33%). Only 3 articles described methods for sample size estimation (5%), and none of the authors explicitly stated the data inclusion or exclusion criteria. Blinding was reported in 38 papers (63.33%). Individualized scores for each study included are available at https://osf.io/pgrhq.

Reporting quality assessment of the included studies. The reporting quality assessment was performed by two independent investigators based on the criteria by41. Each item was scored as yes or no, meaning that the item is either reported or not, respectively. Classification is given as the percentage of assessed studies (n = 60) presenting each score.
Meta-analysis
Distance
The meta-analysis included 61 comparisons from 12 independent studies. The total of animals used as controls was 1112, whereas the exposure individuals counted 2045. The highest concentration of fungicide in the meta-analysis was 20 mg/L for triadimefon63, while the lowest was 0.0001 mg/L for mepanipyrim, ziram, tiophanate, captan, and boscalid26,64.
The overall analysis showed that exposed animals present a lower distance traveled as compared to controls (SMD −0.44 [−0.74; −0.13], p = 0.0055, Fig. 3). The estimated heterogeneity was considered high, with an I2 = 80%, a τ2 = 0.88, and a Q = 300.1 (df = 60, p < 0.01). When calculating strictly for the developmental stage of the larvae, there was a significant effect of the fungicides on decreasing the distance traveled (SMD −0.44 [−0.83; −0.05], p = 0.03, Fig. 3). The heterogeneity was still considered high for this subgroup, with an I2 = 84%, a τ2 = 1.21, and a Q = 284.48 (p < 0.01). Similarly, analyzing the adults subgroup, there was a significant effect of the exposure to fungicides on decreasing the distance traveled (SMD −0.55 [−0.89; −0.21], p < 0.01, Fig. 3). Unlike the larvae, the heterogeneity was considered low, with an I2 = 5%, a τ2 = 0.07, and a Q = 13.72 (p = 0.39). The difference between subgroups was not significant (p = 0.68), indicating that the developmental stage is not a direct moderator for this outcome.

The effect of exposure to fungicides on distance traveled in zebrafish. Subgroup analyses were based on the developmental stage (either larva or adult). Data are presented as Hedges’ G standardized mean differences (SMD) and 95% confidence intervals.
The result from the meta-analysis of distance using only fungicides of the triazole group was similar, and a decrease in distance was observed (Fig. S2).
Spontaneous movements
The meta-analysis comprised 64 comparisons from 13 independent studies. The total of embryos used as controls was 190, and the exposure individuals counted 670. The highest fungicide concentration in the meta-analysis was 145.89 mg/L for cyproconazole65, while the lowest was 0.0001 mg/L for cyprodinil66. All the experiments performed the outcome assessment at 24 h of exposure, except for one (48 h).
The overall analysis showed that fungicide exposure had no significant effect on the number of spontaneous movements (SMD −0.16 [−0.67; 0.34], p = 0.5265, Fig. 4). The estimated heterogeneity was considered moderate, with an I2 = 74%, a τ2 = 1.86, and a Q = 243.19 (df = 63, p < 0.01).

The effect of exposure to fungicides on spontaneous movements in zebrafish. Data are presented as Hedges’ G standardized mean differences (SMD) and 95% confidence intervals.
The result from the meta-analysis of spontaneous movements using only fungicides of the anilide or triazole groups was similar, and no significant effects were observed (Fig. S3 and S4, respectively).
The meta-regression of both outcomes showed no significant correlation of the concentration with the effects (Fig. S5 and S6). Meta-regressions excluding studies from67 (distance)65, and68 (spontaneous movements), maintained no significant correlation (Fig. S7 and S8).
Additional information regarding the meta-analysis can be found at https://osf.io/hdu5c/.
Publication bias
Visual inspection of the funnel plot for the distance outcome showed an asymmetrical distribution of the studies (Fig. 5a). Trim and fill analysis for distance imputed 4 studies to the meta-analysis. The overall effect of the fungicide exposure was no longer significant for this outcome when imputing potentially unpublished data (SMD −0.29 [−0.66, 0.08], p = 0.1252).

Funnel plot including studies analyzed within distance (a) and spontaneous movements (b) outcomes. Each gray circle represents a single comparison. Hollow circles represent imputed studies in the trim and fill analysis. The vertical line represents the overall effect size, and the triangular region represents the 95% confidence interval. Shaded areas represent the interval for statistically significant effects.
For spontaneous movements, the funnel plot also demonstrated an asymmetrical distribution (Fig. 5b). Trim and fill analysis for this outcome imputed 20 studies to the meta-analysis, and the overall effect of fungicide exposure remained not significant (SMD 0.64 [−0.02, 1.29], p = 0.0568).
Egger’s regression test indicated publication bias only for spontaneous movements, which showed a p < 0.0001 (for distance, p = 0.4120) (Table S1).
Sensitivity analysis
The leave-one-out analysis for distance revealed that none of the comparisons significantly modified the meta-analysis result (Fig. 6a). The overall effect and heterogeneity remained close to the original value. However, to confirm that any isolated study is skewing the results, we performed another meta-analysis, excluding all the comparisons from the study by67. This study showed unusually high SMD in the forest plot, and the omission of their experiments in the leave-one-out analysis altered the overall effect direction. The significant overall effect was sustained (SMD −0.31 [−0.54; −0.08] (Fig. S9).

Sensitivity analyses for studies for distance (a) and spontaneous movements (b) outcomes. Data are presented as Hedges’ G standardized mean differences (SMD) and 95% confidence intervals.
The leave-on-out analysis for spontaneous movements showed that omitting comparisons did not significantly modify the meta-analysis original result (Fig. 6b). We also ran the meta-analysis without 2 studies:65,68. In the forest plot, these studies showed atypically high SMD, and omitting their experiments in the leave-one-out analysis changed the overall effect direction. Although the direction of the effect changed, it was still not significant (SMD 0.22 [−021; 066]) (Fig. S10).
Discussion
This work aimed to evaluate and synthesize the neurobehavioral effects of fungicide exposure in zebrafish through a systematic review and meta-analysis. As main findings, we can highlight that fungicides cause a decrease in distance traveled by larval and adult zebrafish; no effect was observed on spontaneous movements of embryos.
The locomotor behavior was the category most frequently assessed in the included studies. Along with distance traveled, velocity was also commonly reported. It is important to emphasize that a decreased distance traveled or velocity does not necessarily imply toxicity, as a substance may have a sedative effect. However, even if not directly related to toxicity or locomotor damage, altered locomotion poses a risk to organisms as it impacts their ability to forage, reproduce, and escape predators69. These data should be observed together with the neurochemical outcomes, which were also consistently investigated and are linked to behavioral variation. The included studies frequently reported altered outcomes related to enzymatic activity, some involved in locomotion (AChE) and oxidative status (GST, SOD, GPx, among others), which are possible mechanisms for reduced locomotor behavior. Few included studies reported investigations of behavioral domains other than locomotor (9), and even so, it was limited to anxiety-fear-related and aggressive behavior, revealing a gap in the literature. The lack of standardized protocols or unpublished negative results could explain this observation70.
The overall high heterogeneity observed in the meta-analysis for distance traveled can be attributed to several sources. The experimental conditions, from rearing until exposure and tests, are extremely variable between laboratories. The researchers employed many protocols, including distinct durations of exposure, frequency of solution renewal, number of coexposed animals, age of the fish, type, and test apparatus. When considering the subgroup analysis, studies with adults had a lower heterogeneity than those performed at the larval stage. Even though fewer adult studies were included, we can indeed verify more uniformity between the protocols of these experiments, mostly during the outcome assessment. Therefore, this similarity can explain the low heterogeneity of this subgroup.
Interestingly, there was no significant difference between the subgroups, indicating that the developmental stage of the animals does not significantly impact the effect of fungicides on the distance traveled. Despite the different locomotor mechanisms exhibited by adults and larvae69, it suggests that fungicide exposure consistently affects both subgroups.
On the other hand, the heterogeneity of the outcome of spontaneous movements was considered moderate. Unlike the distance traveled, the spontaneous movements can be measured in a single developmental stage: the embryo, generally at 24 h post-fertilization (hpf). Consequently, the age of the animals can be excluded as a potential source of heterogeneity, which helps to explain why the heterogeneity did not reach the highest level.
The reporting quality analysis showed a high percentage of negative answers, especially regarding “sample size estimation” and “inclusion or exclusion criteria”. None of the authors explicitly stated previously determined parameters for the eligibility of the data. The result from this evaluation indicates that the conclusions of this review should be interpreted with caution since the report of the included studies presents considerable uncertainty. This lack of methodological information has been recognized as one of the main reasons behind the reproducibility crisis in preclinical research71. Aiming to improve the quality of the studies, guidelines for the research report with animals have been developed in the last years72; however, it is a multifaceted problem that demands complex and long-term solutions73.
Trim and fill analysis for distance imputed 4 studies into the meta-analysis, resulting in no overall significant effect. This fact suggests the presence of missing studies with null and/or significant results74. The unpublished data may have influenced the previously observed significant effect, revealing a potential bias towards the publication of studies only with significant findings in which fungicide exposure decreases locomotion. However, Egger’s test suggests no evidence of publication bias.
Despite the input of 20 studies in the trim and fill analysis for spontaneous movements, it did not alter the non-significant overall effect found in the meta-analysis. This indicates that publication bias may not explain the observed non-significance. However, it is important to note that the significant result obtained from Egger’s test indicates the presence of potential publication bias. The Egger’s test suggests a tendency to publish studies with significant results, which could skew the meta-analysis. Although the trim and fill analysis did not change the overall effect, the imputed studies may impact the precision and confidence interval of the effect estimate. There is an important role of selective publishing in the misinterpretation of a meta-analysis75, highlighting the need for new practices regarding the publication of non-significant results. Even if this represents a complex, deep-rooted issue that requires a change in the whole culture of publishing scientific data, some authors have been raising this discussion and proposing alternatives76,77,78,79. However, the results of our publication bias analysis should be interpreted with caution, as our funnel plots were based on SMD versus standard error (SE). Although this method is standard practice in the field, it may introduce distortion and overestimate the existence of publication bias, as demonstrated empirically by Zwetsloot et al.80.
The sensitivity analysis indicated that the meta-analysis results were not significantly influenced by any particular study or set of studies, suggesting that the overall effect size is robust and reliable. This finding supports the validity of the meta-analytic conclusions and can increase the confidence in the reliability of the results. However, the reliability of each comparison could not be determined due to poor reporting practices and a general lack of protocol preregistration.
One limitation of this study was the inclusion of only studies that used analytical-grade fungicides while excluding those involving commercial formulations and fungicide mixtures. This exclusion was necessary to isolate the specific effects of individual chemicals and ensure more accurate conclusions. Although this approach may be less realistic, it enhances the precision of the findings. Additionally, we did not restrict the inclusion criteria to studies involving exposure to environmentally relevant concentrations, as this would severely reduce the number of eligible articles, making it impossible to conduct a comprehensive meta-analysis. Another significant limitation worth highlighting is the potential inclusion of fraudulent data, which becomes evident when implausible results are observed. While various tools and techniques exist to perform statistical checks and verify data integrity, it is important to note that there is currently no foolproof method to confirm whether a study is fraudulent or not definitively. This task becomes even more challenging without direct access to the data.
Our results reinforce the effects of these chemicals, with their misuse representing a threat to the ecosystems. Since we depend on the affected environment, its contamination is an alert to public health. Besides that, we confirm the demand for well-designed studies with greater clarity of report on this topic. The authors should clearly state key elements such as sample size, sample size estimation, data inclusion or exclusion criteria, and blinding. Some available tools, like preregistration of study protocols and adherence to animal studies reporting guidelines such as the ARRIVE72, could be useful. Compliance with specific reporting guidelines for ecotoxicological studies as the “Criteria for Reporting and evaluating Ecotoxicity Data” (CRED)81 is also highly encouraged. In addition, standardization of behavioral tests could enable more comprehensive meta-analyses. These recommendations can lead to more reliable conclusions and contribute to effectively monitoring environmental pollution.
Data availability
All data are available at Open Science Framework (https://osf.io/hdu5c/).
References
-
FAO & WHO. Manual on the development and use of FAO and WHO specifications for chemical pesticides. (FAO, WHO, 2022). https://doi.org/10.4060/cb8401en.
-
Sharma, A. et al. Worldwide pesticide usage and its impacts on ecosystem. SN Appl. Sci. 1, 1446 (2019).
Google Scholar
-
FAOSTAT. Statistics Division of the Food and Agriculture Organization of the United Nations. https://www.fao.org/faostat/en/#data/RP/visualize (2023).
-
Maggi, F., Tang, F. H. M., la Cecilia, D. & McBratney, A. PEST-CHEMGRIDS, global gridded maps of the top 20 crop-specific pesticide application rates from 2015 to 2025. Sci. Data 6, 170 (2019).
Google Scholar
-
Pignati, W. A. et al. Spatial distribution of pesticide use in Brazil: A strategy for Health Surveillance. Cienc. Saude Coletiva 22, 3281–3293 (2017).
Google Scholar
-
Tudi, M. et al. Agriculture development, pesticide application and its impact on the environment. Int. J. Environ. Res. Public. Health 18, 1112 (2021).
Google Scholar
-
Pimentel, D. Amounts of pesticides reaching target pests: Environmental impacts and ethics. J. Agric. Environ. Ethics 8, 17–29 (1995).
Google Scholar
-
Beketov, M. A., Kefford, B. J., Schäfer, R. B. & Liess, M. Pesticides reduce regional biodiversity of stream invertebrates. Proc. Natl. Acad. Sci. USA 110, 11039–11043 (2013).
Google Scholar
-
Clasen, B. et al. Bioaccumulation and oxidative stress caused by pesticides in Cyprinus carpio reared in a rice-fish system. Sci. Total Environ. 626, 737–743 (2018).
Google Scholar
-
McMahon, T. A. et al. Fungicide-induced declines of freshwater biodiversity modify ecosystem functions and services. Ecol. Lett. 15, 714–722 (2012).
Google Scholar
-
Akoto, O., Azuure, A. A. & Adotey, K. D. Pesticide residues in water, sediment and fish from Tono Reservoir and their health risk implications. SpringerPlus 5, 1849 (2016).
Google Scholar
-
Albuquerque, A. F. et al. Pesticides in Brazilian freshwaters: a critical review. Environ. Sci. Process. Impacts 18, 779–787 (2016).
Google Scholar
-
de Araújo, E. P., Caldas, E. D. & Oliveira-Filho, E. C. Pesticides in surface freshwater: a critical review. Environ. Monit. Assess. 194, 452 (2022).
Google Scholar
-
Xie, W., Zhao, J., Zhu, X., Chen, S. & Yang, X. Pyrethroid bioaccumulation in wild fish linked to geographic distribution and feeding habit. J. Hazard. Mater. 430, 128470 (2022).
Google Scholar
-
Delgado-Blanca, I., Ruiz-Medina, A. & Ortega-Barrales, P. Novel sequential separation and determination of a quaternary mixture of fungicides by using an automatic fluorimetric optosensor. Food Addit. Contam. Part Chem. Anal. Control Expo. Risk Assess. 36, 278–288 (2019).
-
Zubrod, J. P. et al. Fungicides: An overlooked pesticide class?. Environ. Sci. Technol. 53, 3347–3365 (2019).
Google Scholar
-
Beckerman, J., Palmer, C., Tedford, E. & Ypema, H. Fifty Years of Fungicide Development, Deployment, and Future Use. Phytopathology® 113, 694–706 (2023).
-
Richardson, J. R., Fitsanakis, V., Westerink, R. H. S. & Kanthasamy, A. G. Neurotoxicity of pesticides. Acta Neuropathol. (Berl.) 138, 343–362 (2019).
Google Scholar
-
Bakkers, J. Zebrafish as a model to study cardiac development and human cardiac disease. Cardiovasc. Res. 91, 279–288 (2011).
Google Scholar
-
Dai, Y.-J. et al. Zebrafish as a model system to study toxicology. Environ. Toxicol. Chem. 33, 11–17 (2014).
Google Scholar
-
Howe, K. et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 496, 498–503 (2013).
Google Scholar
-
Fitzgerald, J. A., Könemann, S., Krümpelmann, L., Županič, A. & Vom Berg, C. Approaches to Test the Neurotoxicity of Environmental Contaminants in the Zebrafish Model: From Behavior to Molecular Mechanisms. Environ. Toxicol. Chem. 40, 989–1006 (2021).
Google Scholar
-
Jia, M. et al. Developmental toxicity and neurotoxicity of penconazole enantiomers exposure on zebrafish (Danio rerio). Environ. Pollut. Barking Essex 1987 267, 115450 (2020).
-
Jiang, J. et al. Effects of difenoconazole on hepatotoxicity, lipid metabolism and gut microbiota in zebrafish (Danio rerio). Environ. Pollut. Barking Essex 1987 265, 114844 (2020).
-
Meng, Y. et al. Exposure to pyrimethanil induces developmental toxicity and cardiotoxicity in zebrafish. Chemosphere 255, 126889 (2020).
Google Scholar
-
Shen, C., Zhou, Y., Tang, C., He, C. & Zuo, Z. Developmental exposure to mepanipyrim induces locomotor hyperactivity in zebrafish (Danio rerio) larvae. Chemosphere 256, 127106 (2020).
Google Scholar
-
Teng, M. et al. Chronic exposure of zebrafish (Danio rerio) to flutolanil leads to endocrine disruption and reproductive disorders. Environ. Res. 184, 109310 (2020).
Google Scholar
-
Wang, H. et al. Characterization of boscalid-induced oxidative stress and neurodevelopmental toxicity in zebrafish embryos. Chemosphere 238, 124753 (2020).
Google Scholar
-
Gonçalves, Í. F. S. et al. Toxicity testing of pesticides in zebrafish—a systematic review on chemicals and associated toxicological endpoints. Environ. Sci. Pollut. Res. 27, 10185–10204 (2020).
Google Scholar
-
Lushchak, V. I., Matviishyn, T. M., Husak, V. V., Storey, J. M. & Storey, K. B. Pesticide toxicity: a mechanistic approach. EXCLI J. 17, 1101–1136 (2018).
Google Scholar
-
Hamm, J. T. et al. Characterizing sources of variability in zebrafish embryo screening protocols. ALTEX 36, 103–120 (2019).
Google Scholar
-
Yanicostas, C. & Soussi-Yanicostas, N. SDHI fungicide toxicity and associated adverse outcome pathways: What can Zebrafish tell us?. Int. J. Mol. Sci. 22, 12362 (2021).
Google Scholar
-
Frommlet, F. Improving reproducibility in animal research. Sci. Rep. 10, 19239 (2020).
Google Scholar
-
Gerlai, R. Reproducibility and replicability in zebrafish behavioral neuroscience research. Pharmacol. Biochem. Behav. 178, 30–38 (2019).
Google Scholar
-
Reis, C. G. et al. Neurobehavioral effects of fungicides in zebrafish: Protocol https://doi.org/10.17605/OSF.IO/F2D38 (2021).
-
Page, M. J. et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 372, n71 (2021).
Google Scholar
-
Ouzzani, M., Hammady, H., Fedorowicz, Z. & Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 5, 210 (2016).
Google Scholar
-
AERU. Pesticide Properties Database. Agriculture and Environment Research Unit (AERU) at the University of Hertfordshire. http://sitem.herts.ac.uk/aeru/ppdb/en/atoz_fung.htm (2022).
-
van Eck, N. J. & Waltman, L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 84, 523–538 (2010).
Google Scholar
-
van Eck, N. J. & Waltman, L. VOS: A New Method for Visualizing Similarities Between Objects. in Advances in Data Analysis (eds. Decker, R. & Lenz, H.-J.) 299–306 (Springer, 2007). https://doi.org/10.1007/978-3-540-70981-7_34.
-
Landis, S. C. et al. A call for transparent reporting to optimize the predictive value of preclinical research. Nature 490, 187–191 (2012).
Google Scholar
-
McGuinness, L. A. & Higgins, J. P. T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods 12, 55–61 (2021).
Google Scholar
-
Vesterinen, H. M. et al. Meta-analysis of data from animal studies: A practical guide. J. Neurosci. Methods 221, 92–102 (2014).
Google Scholar
-
Statistical Methods for Meta-Analysis. (Academic Press, 1985).
-
Higgins, J. P. T. et al. Cochrane handbook for systematic reviews of interventions. (John Wiley & Sons, 2019).
-
Bakbergenuly, I., Hoaglin, D. C. & Kulinskaya, E. Estimation in meta-analyses of mean difference and standardized mean difference. Stat. Med. 39, 171–191 (2020).
Google Scholar
-
Balduzzi, S., Rücker, G. & Schwarzer, G. How to perform a meta-analysis with R: a practical tutorial. Evid. Based Ment. Health 22, 153–160 (2019).
Google Scholar
-
Wilkinson, L. ggplot2: Elegant graphics for data analysis by WICKHAM. H. Biometrics 67, 678–679 (2011).
Google Scholar
-
Higgins, J. P. T. & Thompson, S. G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 21, 1539–1558 (2002).
Google Scholar
-
Cochran, W. G. Some methods for strengthening the common χ2 tests. Biometrics 10, 417–451 (1954).
Google Scholar
-
Veroniki, A. A. et al. Methods to estimate the between-study variance and its uncertainty in meta-analysis. Res. Synth. Methods 7, 55–79 (2016).
Google Scholar
-
Viechtbauer, W. Bias and efficiency of meta-analytic variance estimators in the random-effects model. J. Educ. Behav. Stat. 30, 261–293 (2005).
Google Scholar
-
Knapp, G. & Hartung, J. Improved tests for a random effects meta-regression with a single covariate. Stat. Med. 22, 2693–2710 (2003).
Google Scholar
-
Richardson, M., Garner, P. & Donegan, S. Interpretation of subgroup analyses in systematic reviews: A tutorial. Clin. Epidemiol. Glob. Health 7, 192–198 (2019).
Google Scholar
-
Viechtbauer, W., López-López, J. A., Sánchez-Meca, J. & Marín-Martínez, F. A comparison of procedures to test for moderators in mixed-effects meta-regression models. Psychol. Methods 20, 360–374 (2015).
Google Scholar
-
Duval, S. & Tweedie, R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 56, 455–463 (2000).
Google Scholar
-
Egger, M., Davey Smith, G., Schneider, M. & Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634 (1997).
Google Scholar
-
Miller, R. G. The Jackknife—A review. Biometrika 61, 1–15 (1974).
Google Scholar
-
Andrade, C. Mean Difference, Standardized Mean Difference (SMD), and Their Use in Meta-Analysis: As Simple as It Gets. J. Clin. Psychiatry 81, 20f13681 (2020).
-
Hill, B. N. et al. Inconsistencies in variable reporting and methods in larval zebrafish behavioral assays. Neurotoxicol. Teratol. 96, 107163 (2023).
Google Scholar
-
OECD. Test No. 203: Fish, Acute Toxicity Test. (Organisation for Economic Co-operation and Development, 2019).
-
OECD. Test No. 236: Fish Embryo Acute Toxicity (FET) Test. (Organisation for Economic Co-operation and Development, 2013).
-
Paredes-Zúñiga, S., Trost, N., De la Paz, J. F., Alcayaga, J. & Allende, M. L. Behavioral effects of triadimefon in zebrafish are associated with alterations of the dopaminergic and serotonergic pathways. Prog. Neuropsychopharmacol. Biol. Psychiatry 92, 118–126 (2019).
Google Scholar
-
Forner-Piquer, I. et al. Varying modalities of perinatal exposure to a pesticide cocktail elicit neurological adaptations in mice and zebrafish. Environ. Pollut. 278, 116755 (2021).
Google Scholar
-
Cao, F. et al. Developmental toxicity of the triazole fungicide cyproconazole in embryo-larval stages of zebrafish (Danio rerio). Environ. Sci. Pollut. Res. 26, 4913–4923 (2019).
Google Scholar
-
Tang, C. et al. Exposure to the AhR agonist cyprodinil impacts the cardiac development and function of zebrafish larvae. Ecotoxicol. Environ. Saf. 201, 110808 (2020).
Google Scholar
-
Li, H. et al. Mitochondrial dysfunction-based cardiotoxicity and neurotoxicity induced by pyraclostrobin in zebrafish larvae. Environ. Pollut. 251, 203–211 (2019).
Google Scholar
-
Yang, Y. et al. Toxic effects of thifluzamide on zebrafish (Danio rerio). J. Hazard. Mater. 307, 127–136 (2016).
Google Scholar
-
Berg, E. M., Björnfors, E. R., Pallucchi, I., Picton, L. D. & El Manira, A. Principles governing locomotion in vertebrates: Lessons From Zebrafish. Front. Neural Circuits 12, 73 (2018).
Google Scholar
-
Sterne, J. A. C. et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 343, d4002 (2011).
Google Scholar
-
Samsa, G. & Samsa, L. A Guide to Reproducibility in Preclinical Research. Acad. Med. J. Assoc. Am. Med. Coll. 94, 47–52 (2019).
Google Scholar
-
Sert, N. P. du et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLOS Biol. 18, e3000410 (2020).
-
Begley, C. G. & Ioannidis, J. P. A. Reproducibility in science: Improving the standard for basic and preclinical research. Circ. Res. 116, 116–126 (2015).
Google Scholar
-
Shi, L. & Lin, L. The trim-and-fill method for publication bias: practical guidelines and recommendations based on a large database of meta-analyses. Medicine (Baltimore) 98, e15987 (2019).
-
van der Worp, H. B. et al. Can animal models of disease reliably inform human studies?. PLOS Med. 7, e1000245 (2010).
Google Scholar
-
Ahmed, A. et al. The future of academic publishing. Nat. Hum. Behav. 7, 1021–1026 (2023).
Google Scholar
-
Bernard, R. et al. fiddle: A tool to combat publication bias by getting research out of the file drawer and into the scientific community. Clin. Sci. 134, 2729–2739 (2020).
Google Scholar
-
Laitin, D. D. et al. Reporting all results efficiently: A RARE proposal to open up the file drawer. Proc. Natl. Acad. Sci. 118, e2106178118 (2021).
Google Scholar
-
Dirnagl, U. & Lauritzen, M. Fighting publication bias: Introducing the negative results section. J. Cereb. Blood Flow Metab. 30, 1263–1264 (2010).
Google Scholar
-
Zwetsloot, P.-P. et al. Standardized mean differences cause funnel plot distortion in publication bias assessments. eLife 6, e24260 (2017).
-
Moermond, C. T. A., Kase, R., Korkaric, M. & Ågerstrand, M. CRED: Criteria for reporting and evaluating ecotoxicity data. Environ. Toxicol. Chem. 35, 1297–1309 (2016).
Google Scholar
-
Domingues, I. et al. Prochloraz effects on biomarkers activity in zebrafish early life stages and adults. Environ. Toxicol. 28, 155–163 (2013).
Google Scholar
-
Fitzmaurice, A. G. et al. Aldehyde dehydrogenase inhibition as a pathogenic mechanism in Parkinson disease. Proc. Natl. Acad. Sci. 110, 636–641 (2013).
Google Scholar
-
Mu, X. et al. Evaluation of acute and developmental effects of difenoconazole via multiple stage zebrafish assays. Environ. Pollut. Barking Essex 1987(175), 147–157 (2013).
Google Scholar
-
Andrade, T. S. et al. Carbendazim exposure induces developmental, biochemical and behavioural disturbance in zebrafish embryos. Aquat. Toxicol. Amst. Neth. 170, 390–399 (2016).
Google Scholar
-
Jin, Y. et al. The fungicide imazalil induces developmental abnormalities and alters locomotor activity during early developmental stages in zebrafish. Chemosphere 153, 455–461 (2016).
Google Scholar
-
Lulla, A. et al. Neurotoxicity of the Parkinson disease-associated pesticide ziram is synuclein-dependent in zebrafish embryos. Environ. Health Perspect. 124, 1766–1775 (2016).
Google Scholar
-
Mu, X. et al. The developmental effect of difenoconazole on zebrafish embryos: A mechanism research. Environ. Pollut. Barking Essex 1987(212), 18–26 (2016).
Google Scholar
-
Yang, Y. et al. Toxic effects of bromothalonil and flutolanil on multiple developmental stages in Zebrafish. Bull. Environ. Contam. Toxicol. 97, 91–97 (2016).
Google Scholar
-
Altenhofen, S. et al. Tebuconazole alters morphological, behavioral and neurochemical parameters in larvae and adult zebrafish (Danio rerio). Chemosphere 180, 483–490 (2017).
Google Scholar
-
De la Paz, J. F., Beiza, N., Paredes-Zúñiga, S., Hoare, M. S. & Allende, M. L. Triazole fungicides inhibit zebrafish hatching by blocking the secretory function of hatching gland cells. Int. J. Mol. Sci. 18, 710 (2017).
Google Scholar
-
da Costa-Silva, D. G. et al. N-acetylcysteine inhibits Mancozeb-induced impairments to the normal development of zebrafish embryos. Neurotoxicol. Teratol. 68, 1–12 (2018).
Google Scholar
-
Fan, Y. et al. Developmental toxicity and inhibition of the fungicide hymexazol to melanin biosynthesis in zebrafish embryos. Pestic. Biochem. Physiol. 147, 139–144 (2018).
Google Scholar
-
Li, H. et al. Developmental toxicity and potential mechanisms of pyraoxystrobin to zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 151, 1–9 (2018).
Google Scholar
-
Qian, L. et al. Mechanisms of developmental toxicity in zebrafish embryos (Danio rerio) induced by boscalid. Sci. Total Environ. 634, 478–487 (2018).
Google Scholar
-
Teng, M. et al. Metabolomics and transcriptomics reveal the toxicity of difenoconazole to the early life stages of zebrafish (Danio rerio). Aquat. Toxicol. Amst. Neth. 194, 112–120 (2018).
Google Scholar
-
Teng, M. et al. Effects of the bioconcentration and parental transfer of environmentally relevant concentrations of difenoconazole on endocrine disruption in zebrafish (Danio rerio). Environ. Pollut. 233, 208–217 (2018).
Google Scholar
-
Wang, X. H. et al. Fluazinam impairs oxidative phosphorylation and induces hyper/hypo-activity in a dose specific manner in zebrafish larvae. Chemosphere 210, 633–644 (2018).
Google Scholar
-
Cao, F. et al. Developmental toxicity of the fungicide ziram in zebrafish (Danio rerio). Chemosphere 214, 303–313 (2019).
Google Scholar
-
Cao, F. et al. Developmental neurotoxicity of maneb: Notochord defects, mitochondrial dysfunction and hypoactivity in zebrafish (Danio rerio) embryos and larvae. Ecotoxicol. Environ. Saf. 170, 227–237 (2019).
Google Scholar
-
Perez-Rodriguez, V., Souders, C. L., Tischuk, C. & Martyniuk, C. J. Tebuconazole reduces basal oxidative respiration and promotes anxiolytic responses and hypoactivity in early-staged zebrafish (Danio rerio). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 217, 87–97 (2019).
-
Qian, L. et al. Effects of penthiopyrad on the development and behaviour of zebrafish in early-life stages. Chemosphere 214, 184–194 (2019).
Google Scholar
-
Souders, C. L. et al. Sub-lethal effects of the triazole fungicide propiconazole on zebrafish (Danio rerio) development, oxidative respiration, and larval locomotor activity. Neurotoxicol. Teratol. 74, 106809 (2019).
Google Scholar
-
Teng, M. et al. Effect of Propiconazole on the Lipid Metabolism of Zebrafish Embryos (Danio rerio). J. Agric. Food Chem. 67, 4623–4631 (2019).
Google Scholar
-
Tian, S. et al. Toxicity effects in zebrafish embryos (Danio rerio) induced by prothioconazole. Environ. Pollut. 255, 113269 (2019).
Google Scholar
-
Valadas, J. et al. Propiconazole induces abnormal behavior and oxidative stress in zebrafish. Environ. Sci. Pollut. Res. 26, 27808–27815 (2019).
Google Scholar
-
Wang, H. et al. Toxic effects of oxine-copper on development and behavior in the embryo-larval stages of zebrafish. Aquat. Toxicol. Amst. Neth. 210, 242–250 (2019).
Google Scholar
-
Yang, Y. et al. Dysregulation of circadian rhythm in zebrafish (Danio rerio) by thifluzamide: Involvement of positive and negative regulators. Chemosphere 235, 280–287 (2019).
Google Scholar
-
Yang, Y. et al. Flutolanil affects circadian rhythm in zebrafish (Danio rerio) by disrupting the positive regulators. Chemosphere 228, 649–655 (2019).
Google Scholar
-
Zhou, Y., Chen, X., Teng, M., Zhang, J. & Wang, C. Toxicity effects of captan on different life stages of zebrafish (Danio rerio). Environ. Toxicol. Pharmacol. 69, 80–85 (2019).
Google Scholar
-
Hussain, A. et al. Multiple screening of pesticides toxicity in zebrafish and daphnia based on locomotor activity alterations. Biomolecules 10, 1224 (2020).
Google Scholar
-
Kumar, N. et al. Developmental toxicity in embryo-larval zebrafish (Danio rerio) exposed to strobilurin fungicides (azoxystrobin and pyraclostrobin). Chemosphere 241, 124980 (2020).
Google Scholar
-
Liu, X., Zhang, R. & Jin, Y. Differential responses of larval zebrafish to the fungicide propamocarb: Endpoints at development, locomotor behavior and oxidative stress. Sci. Total Environ. 731, 139136 (2020).
Google Scholar
-
Pang, S. et al. Myclobutanil developmental toxicity, bioconcentration and sex specific response in cholesterol in zebrafish (Denio rerio). Chemosphere 242, 125209 (2020).
Google Scholar
-
Souders, C. L. et al. Investigation into the sub-lethal effects of the triazole fungicide triticonazole in zebrafish (Danio rerio) embryos/larvae. Environ. Toxicol. 35, 254–267 (2020).
Google Scholar
-
Vasamsetti, B. M. K., Kim, N.-S., Chon, K. & Park, H.-H. Teratogenic and developmental toxic effects of etridiazole on zebrafish (Danio rerio) embryos. Appl. Biol. Chem. 63, 80 (2020).
Google Scholar
-
Zhang, X., Zhang, P., Perez-Rodriguez, V., Souders, C. L. & Martyniuk, C. J. Assessing the toxicity of the benzamide fungicide zoxamide in zebrafish (Danio rerio): Towards an adverse outcome pathway for beta-tubulin inhibitors. Environ. Toxicol. Pharmacol. 78, 103405 (2020).
Google Scholar
-
Barreto, A., Santos, J., Amorim, M. J. B. & Maria, V. L. Is the Synthetic Fungicide Fosetyl-Al Safe for the Ecotoxicological Models Danio rerio and Enchytraeus crypticus?. Appl. Sci. 11, 7209 (2021).
Google Scholar
-
Brenet, A., Hassan-Abdi, R. & Soussi-Yanicostas, N. Bixafen, a succinate dehydrogenase inhibitor fungicide, causes microcephaly and motor neuron axon defects during development. Chemosphere 265, 128781 (2021).
Google Scholar
-
Fan, R. et al. Individual and synergistic toxic effects of carbendazim and chlorpyrifos on zebrafish embryonic development. Chemosphere 280, 130769 (2021).
Google Scholar
-
Huang, T. et al. Behavioral and developmental toxicity assessment of the strobilurin fungicide fenamidone in zebrafish embryos/larvae (Danio rerio). Ecotoxicol. Environ. Saf. 228, 112966 (2021).
Google Scholar
-
Leandro, L. P. et al. Behavioral changes occur earlier than redox alterations in developing zebrafish exposed to Mancozeb. Environ. Pollut. 268, 115783 (2021).
Google Scholar
-
Li, X. Y. et al. Relative comparison of strobilurin fungicides at environmental levels: Focus on mitochondrial function and larval activity in early staged zebrafish (Danio rerio). Toxicology 452, 152706 (2021).
Google Scholar
-
Lin, H., Lin, F., Yuan, J., Cui, F. & Chen, J. Toxic effects and potential mechanisms of Fluxapyroxad to zebrafish (Danio rerio) embryos. Sci. Total Environ. 769, 144519 (2021).
Google Scholar
-
Paredes-Zúñiga, S., Ormeño, F. & Allende, M. L. Triadimefon triggers circling behavior and conditioned place preference/aversion in zebrafish in a dose dependent manner. Neurotoxicol. Teratol. 86, 106979 (2021).
Google Scholar
-
Pompermaier, A. et al. Water and suspended sediment runoff from vineyard watersheds affecting the behavior and physiology of zebrafish. Sci. Total Environ. 757, 143794 (2021).
Google Scholar
-
Qian, L. et al. Environmentally relevant concentrations of boscalid exposure affects the neurobehavioral response of zebrafish by disrupting visual and nervous systems. J. Hazard. Mater. 404, 124083 (2021).
Google Scholar
-
Tang, C. et al. Long-term exposure to cyprodinil causes abnormal zebrafish aggressive and antipredator behavior through the hypothalamic–pituitary–interrenal axis. Aquat. Toxicol. 241, 106002 (2021).
Google Scholar
-
Wu, A. et al. Developmental toxicity of procymidone to larval zebrafish based on physiological and transcriptomic analysis. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 248, 109081 (2021).
-
Yang, Y. et al. Thifluzamide exposure induced neuro-endocrine disrupting effects in zebrafish (Danio rerio). Arch. Toxicol. 95, 3777–3786 (2021).
Google Scholar
-
Yang, L. et al. Evaluation and comparison of the mitochondrial and developmental toxicity of three strobilurins in zebrafish embryo/larvae. Environ. Pollut. Barking Essex 1987 270, 116277 (2021).
Acknowledgements
We thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, proc. 303343/2020-6), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES), and Pró-Reitoria de Pesquisa (PROPESQ) at Universidade Federal do Rio Grande do Sul (UFRGS) for funding and support. CGR is a recipient of a fellowship from CAPES.
Author information
Authors and Affiliations
Contributions
C.G.R.: conceptualization, data curation, formal analysis, investigation, methodology, project administration, visualization, and writing—original draft; L.M.B.: conceptualization, investigation, methodology, visualization, and writing—review & editing; R.C.: conceptualization, investigation, methodology, and writing—review & editing; M.G.-L.: data curation, formal analysis, methodology, visualization, and writing—review & editing; Q.K.Z.: conceptualization, methodology, and writing—review & editing; S.Z.B.: conceptualization, investigation, and writing—review & editing; A.P.H.: conceptualization, investigation, methodology, supervision, project administration, and writing—review & editing; A.P.: conceptualization, investigation, methodology, supervision, project administration, and writing—review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Information.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
Reprints and Permissions
About this article
Cite this article
Reis, C.G., Bastos, L.M., Chitolina, R. et al. Neurobehavioral effects of fungicides in zebrafish: a systematic review and meta-analysis.
Sci Rep 13, 18142 (2023). https://doi.org/10.1038/s41598-023-45350-6
-
Received: 27 July 2023
-
Accepted: 18 October 2023
-
Published: 24 October 2023
-
DOI: https://doi.org/10.1038/s41598-023-45350-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.