Abstract
Chemotherapy is an effective strategy for mitigating the global challenge of cancer treatment, which often encounters drug resistance and negative side effects. Methylnaphthazarin (MNZ), a natural compound with promising anti-cancer properties, has been underexplored due to its poor aqueous solubility and low selectivity. This study introduces a novel approach to overcome these limitations by developing MNZ-encapsulating liposomes decorated with folate and biotin (F/B-LP-MNZ). This dual-targeting strategy aims to enhance the anti-cancer efficacy and specificity of MNZ delivery. Our innovative F/B-LP-MNZ formulation demonstrated excellent physicochemical properties, stability, and controlled drug release profiles. In vitro studies revealed that MNZ-loaded liposomes attenuate the toxicity associated with free MNZ while F/B-LP-MNZ significantly increased cytotoxicity against HeLa cells, which express high levels of folate and biotin receptors, compared to non-targeted liposomes. Enhanced cellular uptake and improved dynamic flow attachment further confirmed the superior specificity of F/B-LP in targeting cancer cells. Additionally, our results revealed that F/B-LP-MNZ effectively inhibits HeLa cell migration and adhesion through EMT suppression and apoptotic induction, indicating its potential to prevent cancer metastasis. These findings highlight the potential of dual folate and biotin receptors-targeting liposomes as an effective delivery system for MNZ, offering a promising new avenue for targeted cancer therapy.
Introduction
Chemotherapy is a promising systemic treatment for advanced-stage cancers to shrink the tumor size combined with surgery or other treatments. Additionally, the patient can admit therapeutic drugs at home conveniently. However, nearly 90% of failed cases in chemotherapy are because of drug resistance development of tumor cells, resulting in increased cancer invasion and metastatic progression1. Therefore, increasing the dose of drug administration or changing chemotherapeutic drugs for cancer treatment is needed to suppress the metastasis of tumor cells. However, giving the extra dose of chemotherapeutic drugs is not safe for the patient. Also, many chemotherapeutic drugs currently used have a lot of unwanted side effects including hair loss, bone marrow suppression, vomiting, rash, inflammation, and induced reticulate hyperpigmentation2,3. Consequently, exploring novel anti-cancer agents and effective drug delivery systems is ongoing. Cancer-targeting nanocarriers, classified as passive and active targeting, have emerged to promote selectivity and efficient cancer therapy4,5. Passive targeting leverages the natural properties of tumor vasculature to deliver chemotherapeutic drugs more effectively4. This approach can improve the permeability and retention effects of drug by optimizing the size, shape, surface properties (charge and hydrophilicity/ hydrophobicity), and stability of nanoparticles4,5,6,7. Meanwhile, active targeting nanoparticles are functionalized with ligands such as antibodies, peptides, aptamers, or small molecules that have a high affinity for specific receptors on the surface of cancer cells. Receptor-mediated endocytosis facilitates the internalization of nanoparticles, increases specificity, reduces side effects, and protects against lysosomal degradation and clearance4,5,8.
Liposomes emerged as an effective platform for facilitating the delivery of chemotherapeutic agents to target cancer. Liposomes are highly biocompatible, biodegradable, and nonimmunogenic vesicles that can be formulated from natural non-toxic ingredients. The small spherical artificial vesicles of liposomes consist of one or more phospholipid bilayers9. Apart from phospholipids, cholesterol-a sterol found in the eukaryotic cell membrane-is used to fill the gaps between phospholipids to stabilize and prevent the aggregation of liposomes10. Liposomes are one of the promising systems for targeted delivery of numerous chemotherapeutic drugs. Liposome surface modification and functionalization are the significant advantages of the new generation of liposomes, which tremendously improve their stability and efficacy in cancer therapy. For example, introducing polyethylene glycol (PEGylation) to the phospholipids effectively increases liposome stability and delays the clearance of drugs in blood vessels11,12. Conjugation of the phospholipids with specific ligands antibodies or high-affinity binders of cell receptors such as peptides, vitamins, and other molecules can promote the selectivity and recognizability of targeted drug delivery13. Cancer cells are more aggressive and need more nutrients to support their growth, as a result, cancer cells typically have high expression levels of nutrient receptors and protein-associated metastasis14. Therefore, these proteins can be used as protein markers for targeted delivery of anti-cancer agents, for instance, CD4415,16, biotin receptor17,18, folate receptor19,20, and integrins21,22, as shown in Supplementary Table S1.
Methylnaphthazarin (2-methyl-5,8-dihydroxy-1,4-naphthoquinone, MNZ) is a natural constituent found in Nepenthes thorelii, Drosera intermedia, etc23,24. MNZ exhibited strong anticancer activity against HL-60 cells (lung, IC50: 0.64 µM) in which it acts as a thioredoxin reductase inhibitor, resulting in induced oxidative stress-mediated apoptosis of HL-60 cells25. Furthermore, MNZ showed strong antimalarial activity (Plasmodium falciparum T9/94, IC50: 5.79 µM)23. Even though this information emphasized that MNZ can be used as an anticancer agent, the cancer-inhibitory activity of this compound has not been broadly studied.
Hence, this work aimed to develop MNZ-loaded liposomes targeting folate and biotin receptors to enhance the selectivity and precision of cancer treatment of MNZ. Herein, we successfully synthesized MNZ and developed folate/biotin-decorated liposomes loaded with MNZ. The appropriate physicochemical characteristics of liposomes were determined by measuring liposome size, polydispersity index, zeta potential, and long-term stability. We then performed several experiments in both monolayers and 3D spheroids of cancer cells to confirm the improvement of targeted drug delivery to cancer cells including in vitro drug release profiles, cellular cytotoxicity, and drug uptake in static and dynamic flow conditions. Furthermore, the effects of folate/biotin-decorated liposomes loaded with MNZ on suppressing cancer metastasis were investigated to confirm the effectiveness of MNZ encapsulation in cancer treatment.
Methods
Materials
2-methylmaleic anhydride and 1,4-benzenediol were purchased from TCI Chemicals (Japan). Coumarin 6 (Cou6) and didodecyldimethylammonium bromide (DDAB) were obtained from Sigma-Aldrich (Saint Louis, MO, USA). DSPE-PEG(2000) Amine, DSPE-PEG(2000) Folate, and DSPE-PEG(2000) Biotin were acquired from Avanti Polar Lipids (Birmingham, AL, USA). Cholesterol-NF was acquired from Cosmeplus (Bangkok, Thailand). Phospholipon 90G was obtained from Cargill Siam (Bangkok, Thailand). MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) and dil (Tetramethylindocarbocyanine Perchlorate) were purchased from Invitrogen (Waltham, MA, USA). The A.R grade of ethyl acetate, dichloromethane, dimethyl sulfoxide (DMSO), hexane, and methanol, and HPLC grade of acetonitrile were purchased from Carlo Erba (Emmendingen, Germany). Aluminum chloride was obtained from Fluka (Buchs, Switzerland). SiliaFlash P60 was purchased from SiliCycle (Quebec, Canada).
Preparation of methylnaphthazarin
The synthesis of methylnaphthazarin (MNZ) was based on the methods of Zhang et al.25. MNZ was synthesized by using 2-methylmaleic anhydride and 1,4-benzenediol as starting materials. The condition and method in detail are illustrated in Section A1 (supplementary information).
Preparation of folate/biotin-decorated liposomes loaded with MNZ
The liposomes were formulated using the thin-film hydration method applied from Wen et al.21 and Wongkhieo et al.26. The compositions used for formulating liposomes-loaded with MNZ (LP-MNZ) and folate/biotin-decorated liposomes-loaded with MNZ (F/B-LP-MNZ) are depicted in Table 1. The detailed methods are illustrated in Section A2 (supplementary information).
Characterization of nanoparticles and their stability
Particle sizes, polydispersity index, and zeta potential of the formulated nanoparticles were measured using dynamic light scattering (DLS) technique at 25 °C with Zetasizer Nano ZX (Malvern Instruments, UK). Long-term stability of liposomes during three months of preservation at 4 and 25 °C was determined by measuring particle sizes, polydispersity index, and zeta potential. The liposome morphology was determined under a transmission electron microscope. Studies of encapsulation efficiency (EE%) and the drug loading capacity (LC%) further proceeded with the detailed methods as illustrated in Section A3 (supplementary information).
In vitro drug release
The release behavior of MNZ from liposomes was performed by using 16 mm diameters SnakeSkin 3.5 K MWCO dialysis tubing (Thermo Fisher Scientific) as previously reported in Wen et al.21. PBS buffer (pH 7.4) and sodium citrate buffer (pH 5.4) were used as media buffers for simulating the physiological condition and tumor acidic microenvironment. The detailed methods are illustrated in Section A4 (supplementary information).
Cell culture
Human cervical carcinoma (HeLa) cells, human embryonic kidney (HEK293T) cells, and human dermal fibroblast (HDFa) cells were acquired from National Nanotechnology Center (NANOTEC, NSTDA, Thailand). All cell lines were cultured in DMEM (Dulbecco’s Modified Eagle Medium) containing 4.5 g/L glucose, L-glutamine, 110 mg/L sodium pyruvate, and supplemented with 10% fetal bovine serum (FBS) and 1% antibiotic–antimycotic drugs (Gibco, USA). Cells were usually grown at 37˚C in the incubator supplied with 5% CO2. Whereas, DMEM-low glucose containing 10% FBS was used for anti-migration assay instead. Trypsin–EDTA (0.25%) was used for trypsinization.
In vitro cell viability assay
MTT assay was performed to determine the cell viability of cell monolayers as described in Wongkhieo et al.26. Whereas, CellTiter-Glo Luminescent Cell Viability Assay Kit (Promega, USA) was used for determining the cell viability of 3D spheroids. The detailed methods are illustrated in Section A6 (supplementary information).
Cellular uptake assay in 2D monolayers
In this experiment, non-targeted liposomes (plain LP) and F/B-decorated liposomes (F/B-LP) loading with fluorescent dye Cou6 were used for assessing intracellular uptake. Cell monolayers of high-folate receptor (FR)/biotin receptor (BR)-expressing (HeLa) cells and low-FR/BR-expressing (HEK293T) cells were incubated with varying concentrations of Cou6-labelled liposomes for 1 h. The detailed methods are illustrated in Section A7.1 (supplementary information).
Cellular uptake and competition test in 3D spheroids
3D HeLa spheroids were formed as described in Section A7.1 (supplementary information). Spheroid cells were treated with varying concentrations of plain LP and F/B-LP loading Cou6 in the presence and absence of 1 mM folic acid in the medium for 1 h. The detailed methods are illustrated in Section A7.2 (supplementary information).
Dynamic flow adhesion of liposomes
HeLa and HDFa cells were cultured onto a 6-well plate until proliferate over 90% confluence. In this study, non-decorated liposomes (plain LP) and F/B-decorated liposomes (F/B-LP) labeling dil fluorescent dye were used to investigate the adhesion of liposomes to the cells under flow conditions. The parallel plate flow chamber fabrication system was set up to mimic wall shear stress in blood vessels as described by Namdee et al.27. The detailed methods are illustrated in Section A8 (supplementary information).
Anti-migration assay
Scratch wound healing assay was conducted to study the inhibition of HeLa cell migration using scratch wound healing assay with culture-insert 4 well in µ-dish 35 mm (Ibidi, USA). The detailed methods are described in Section A9 (supplementary information).
Cell adhesion assay
The capability of F/B-LP-MNZ towards suppressing the adhesion of HeLa cells onto a 48-well cell culture plate was performed according to the illustrated methods in Section A10 (supplementary information).
Real-time PCR (qPCR)
Total RNA was isolated by using TRIzol reagent (Invitrogen) with a common RNA extraction protocol. The reverse transcription was performed by using a High-Capacity RNA-to-cDNA Kit (Applied Biosystems), and real-time qRT-PCR was conducted according to the Applied Biosystems instruction (the Fast SYBR™ Green Master Mix, cat. no. 4385612). The detailed methods and used primers are described in Section A11 (supplementary information).
Statistical analysis
Data presented in this study were reported as either mean ± standard deviation (s.d.) or mean ± standard error of mean (s.e.m.). Statistical comparison was performed using either one-way ANOVA followed by Dunnett’s post hoc analysis or two-way ANOVA followed by Tukey’s post hoc analysis among multiple groups using GraphPad Prism 9 software. Statistically significant differences were considered at p-value < 0.05.
Results and Discussion
Synthesis of methylnaphthazarin
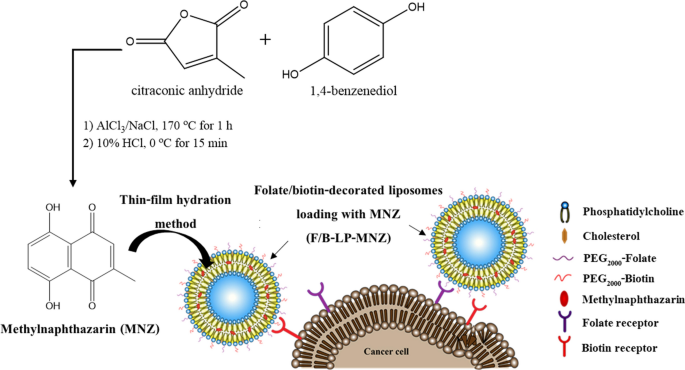
MNZ, a rare natural product, was successfully synthesized. The synthetic scheme is shown in Fig. 1. After purification using column chromatography, the dark red solid of MNZ was obtained about 12% yield from hexane fraction. The chemical structure of MNZ was then characterized by using 1H-NMR and 13C-NMR as shown in supplementary Fig. S1, and it showed a purity of more than 99% when analyzed using HPLC (see supplementary Fig. S2).

Scheme depicting MNZ synthesis and its encapsulation into folate/biotin-decorated liposomes.
MNZ is not the major constituent in plants. A synthetic approach was used to obtain a large amount of MNZ for further studies. Unlike the previous study, the yield of MNZ obtained was slightly lower than previously reported. We observed that the reaction mixture solidified immediately after adding a mixture of starting materials to the molten mixture of AlCl3 and NaCl. However, the reaction mixture reverted to a liquid state after neutralization with 10% HCl.
Preparation of liposomes and physicochemical characterization
To formulate the folate/biotin-decorated liposomes for delivery of MNZ, cationic soy lecithin and surfactant, cholesterol, and DSPE-PEG(2000) conjugated with folate and biotin at the appropriate ratios (Table 1) were utilized in liposome formulations using thin-film hydration method followed by ultrasonication. The obtained liposomes loaded with MNZ had a red color transparent solution as depicted in Fig. S3a (supplementary information). The successful liposome formulations were then confirmed by measuring particle size, polydispersity index, and zeta potential. As indicated in Table 2, the average sizes of LP-Blank, LP-MNZ, and F/B-LP-MNZ were 133.47 ± 1.10, 121.93 ± 1.94, and 110.93 ± 3.04 nm, respectively. All liposomes exhibited narrow size distribution with an average PDI of about 0.25–0.26 and a high positive charge with an average zeta potential of around 45–46 mV. However, the smaller particle sizes of targeted liposomes than non-targeted ones were observed. The presence of targeting ligands like folate and biotin can lead to a more uniform distribution of lipids during the liposome formation process, contributing to the formation of smaller sizes. This phenomenon is similar to the previous reports, where the development of glycyrrhetinic acid and galactose-modified liposomes for co-delivery of curcumin and capsaicin targeting liver cancer showed smaller particle size compared to non-targeted and single-targeted liposomes28. In another study, the formulation of irinotecan-loaded folate-targeted liposomes posed a particle size larger than targeted liposomes29. Folate and biotin are natural water-soluble vitamins. Possibly, the conjugation of folate and biotin in DSPE-PEG2000 chains improves the solubility in the polar and aqueous environments compared to DSPE-PEG2000-Amine. Furthermore, the incorporation of folate and biotin in DSPE-PEG2000 chains may facilitate the small vesicle assembly during liposome formation. Thereby, the presence of folate and biotin can contribute to the overall stability and size reduction by enhancing the solubility of the liposomes and possibly disrupting the fusion to larger lipid vesicles.
The ratio of compositions used for formulating liposomes showed highly efficient encapsulation of MNZ with encapsulation efficiency of 99.32 ± 1.80 and 99.13 ± 2.70% for LP-MNZ and F/B-LP-MNZ, respectively. Loading capacities were 4.15 ± 0.07 and 4.02 ± 0.27% for LP-MNZ and F/B-LP-MNZ, respectively (see Table 2). The morphology of liposomes was further studied. As shown in supplementary Fig. S3d–g, the TEM images of all liposome formulations showed spherical shape and their sizes corresponded with values obtained from DLS measurement.
The selection of appropriate lipids for liposome construction plays a crucial role in enhancing drug delivery efficiency. Based on these results, cationic liposomes were obtained through the combination of soybean phosphatidylcholine and DDAB. The highly positive charge of the liposomes not only increases the repulsive force between particles to prevent the aggregation or agglomeration of nanoparticles but also facilitates targeted drug delivery to cancer cells compared to neutral and anionic liposomes6,30,31,32,33. In some cases, the incorporation of cationic to nanoparticles can attenuate the cytotoxic effect. However, use of excess amounts of cationic lipids in nanomedicine also contributes to the cytotoxicity and inflammation34.
Long-term stability of liposome formulations
The stability of all formulated liposomes during long-term preservation for 3 months at 4 and 25˚C was investigated by measuring particle size, PDI, and zeta potential every month. As presented in supplementary Fig. S4, we observed no significant changes in particle size, PDI, and zeta potential for LP-Blank, LP-MNZ, and F/B-LP-MNZ throughout the 3 months when stored at 4 and 25˚C. Although there were slight variations in the numerical values between preservation at 4 and 25˚C, these liposomes maintained desirable characteristics as nanocarriers, with an average particle size < 300 nm, PDI 30 mV. These findings suggest that the liposome formulation effectively stabilizes liposome integrity and extends their shelf-life by at least 3 months under storage conditions of 4 and 25˚C. However, for a more efficient delay of lipid instability and remodeling, storage at low temperatures under oxygen-free conditions and protection from light is recommended35,36.
In vitro drug release study
One of the crucial hallmarks of cancer is its adaptation to acidosis, which enhances metastasis and confers resistance to cell-mediated immunity and chemotherapeutic drugs. The tumor’s acidic microenvironment arises from the high glycolytic rate of cancer cell metabolism, hypoxia, and blood perfusion deficit37. In this study, we simulated the extracellular pH of tumor and normal tissues to investigate drug release in vitro using PBS at pH 5.4 and 7.4, respectively. As shown in Fig. 2a, the results revealed exponential release profiles of free MNZ within 30 min and gradually constant after that in both conditions of pH 5.4 and 7.4. Conversely, F/B-LP-MNZ exhibited the controlled release patterns of MNZ at both pH levels compared to free MNZ. Furthermore, F/B-LP-MNZ demonstrated a preference for releasing in acidic conditions compared to neutral pH. These findings suggest the potential for drug release to occur more prominently around tumor cells than normal cells due to greater liposomal destabilization and drug diffusion triggered by the lower pH of the cancer microenvironment. There are various mechanisms relating to drug release in acidic microenvironments: one is the protonation/deprotonation of phospholipids alters the electrostatic interactions within the lipid bilayers38, and the second is liposome erosion. These phenomena can ultimately lead to destabilization and increased permeability of the liposomal membrane allowing for the passive diffusion of encapsulated drugs39.

Drug release profiles of free MNZ and its folate/biotin-decorated liposomes (F/B-LP-MNZ) in different pH conditions. (a) Cumulative release profile of free MNZ and F/B-LP-MNZ in microenvironments of tumor (pH 5.4) and normal (pH 7.4) tissues at room temperature using dialysis membrane. (b) Relative cumulative drug release (ratio of F/B-LP-MNZ / free MNZ) quantified from (a). Data are presented as mean ± s.d. (n = 3).
In our study, we observed that the release behavior of free MNZ in an acidic condition (pH 5.4) was higher than in a neutral condition (pH 7.4). This difference can be attributed to the solubility and ionization characteristics of MNZ in varying pH levels. Like many other small molecules, MNZ can exhibit distinct solubility profiles and ionization states depending on the pH. The ionization state of MNZ directly influences its solubility in aqueous solutions by the protonation or deprotonation of functional groups of MNZ altering its solubility and, consequently, its release kinetics. Specifically, under acidic conditions (lower pH), increased protonation of MNZ can enhance its solubility and lead to faster diffusion rates. As reported by Hosoya and team40, demonstrating that 5,8-Dihydroxy-[1,4]-naphthoquinone (MNZ derivative) was protonated in acidic media, while deprotonated in alkaline conditions. However, the literature presents mixed findings regarding the solubility of naphthoquinone at different pH levels. For instance, Linares et al.41 reported increased solubility of a naphthoquinone when the pH of the buffer was raised from pH 3.8 to 8.0, whereas Lide and Milne42 found that 1,4-naphthoquinone was only slightly soluble in weak acids and robustly soluble in sulfuric acid. Given these varied reports, it is clear that both buffer composition and pH are critical factors influencing the MNZ solubility. To address this variability and ensure comparability of drug release behavior under different conditions, we normalized the cumulative drug release values at the two pH levels by converting them to relative cumulative drug release values (the ratio of F/B-LP-MNZ to free MNZ), as depicted in Fig. 2b. The results consistently showed significant differences in the pH-dependent release of MNZ from F/B-LP-MNZ at pH 5.4 compared to pH 7.4, emphasizing the influence of pH on MNZ release kinetics.
In vitro cytotoxicity
To investigate the effects of folate/biotin- and non-decorated liposomes loaded with MNZ on anti-cancer activities, cells representing high expression levels of FR and BR (HeLa)43,44,45 and cells representing low expression levels of FR and BR (HEK293T)45,46,47 were used in this study. Cellular cytotoxicity of free MNZ, LP-Blank, LP-MNZ, and F/B-LP-MNZ was tested with HeLa and HEK293T cells in both monolayer and 3D spheroid cultures. The results of cell viability after 48 h of treatment are presented in Fig. 3. LP-Blank was cytotoxicity control of empty nanocarriers. We found that LP-MNZ and F/B-LP-MNZ at low concentrations are less toxic when compared to free MNZ at the same concentrations in both HeLa and HEK293T cells. The controlled release behavior of drug-loaded liposomes can reduce the rapid drug diffusion and overexposed amount of free MNZ to the cells contributing to lower cytotoxicity. Interestingly, the cell viability of HeLa significantly decreased when treated with F/B-LP-MNZ at concentrations 1.25, 2.5, and 5 µM MNZ compared to LP-MNZ, and no significant differences between free MNZ and F/B-LP-MNZ at 2.5, and 5 µM MNZ (Fig. 3a). Moreover, HeLa cells seem to be more susceptible to free MNZ and F/B-LP-MNZ when compared to HEK293T cells (Fig. 3a,b). Also, the cytotoxic testing against 3D HeLa spheroids showed a correlated trend with testing in the HeLa cell monolayer. F/B-LP-MNZ at concentrations 2.5 and 5 µM MNZ significantly decreased the cell viability of 3D HeLa spheroids when compared to LP-MNZ (Fig. 3c,d). These results suggested that F/B-LP-MNZ at concentrations ranging from 2 to 5 µM MNZ is the appropriate window for cancer treatment.

The effect of free MNZ, LP-Blank, LP-MNZ, and F/B-LP-MNZ on cell viability of high- and low-folate receptor/biotin receptor-expressing cells (HeLa and HEX293T, respectively). HeLa monolayer (a), HEK293T monolayer (b), and 3D HeLa spheroids (d) were incubated with indicated concentrations of free MNZ and liposomes for 48 h. (c) The representative phase-contrast images of 3D HeLa spheroids after 48 h incubation with 2.5 µM of free MNZ and liposomes. Data are presented as mean ± s.d. (n ≥ 3). ** p < 0.01, *** p < 0.001 vs F/B-LP-MNZ. ## p < 0.01 and ### p < 0.001 vs free drug.
Cellular uptake of drug-loaded F/B-LP
In general, nanoparticles enter the cells via four main pathways: clathrin/caveolae-mediated endocytosis, macropinocytosis, and pinocytosis, phagocytosis. The selective permeability of the plasma membrane of cells enables endocytosis pathways based on the size, shape, and surface of nanoparticles8,48. Previous studies reported that nanoparticles with smaller sizes and rods or disc in shape nanoparticles positively alter the uptake of drug49. Additionally, due to the acidic pH of the outer membrane of cancer cells, phospholipids in the cell membrane are arranged differently than the cell membrane of healthy cells, resulting in the outer membrane of the cancer cell becoming cationic liposomes50. Therefore, introducing cationic lipids into nanoparticles could enhance internalization and decrease clearance of drug in cancer cells6,7,31.
This experiment examined the enhancement of cellular uptake of FR/BR-targeting liposomes in HeLa and HEK293T monolayer cells, as well as HeLa spheroids. Figure 4 illustrates the uptake of Cou6-labeled plain liposomes and F/B-liposomes at 50 and 100 ng/mL Cou6 in monolayer cells after 1 h of exposure. The results in 2D monolayers revealed that F/B-LP could enhance the cellular uptake of the drug compared to plain LP in HeLa cells but not in HEK293T cells (Fig. 4b,c).

In vitro cellular uptake in high- and low-FR/BR expressing cells in both monolayer and spheroids. (a) Representative fluorescence images of cellular uptake of Cou6 in monolayer cells of HeLa and HEK293T. Scale bars are 50 µm. Quantitative cellular uptake in HeLa (b) and HEK293T (c) cells analyzed from (a). Cells were incubated with plain LP (grey bars) and F/B-LP (black bars) labeling Cou6 at a 50 ng/mL concentration for 1 h. (d) Representative fluorescence images of cellular uptake and folic acid competition test in 3D HeLa spheroids. Scale bars are 100 µm. (e) Quantitative cellular uptake in HeLa spheroids quantified from (d). 3D spheroids were incubated with plain LP (grey bars) and F/B-LP (black bars) labeling Cou6 at indicated concentrations in the presence and absence of 1 mM folic acid for 1 h. Data are presented as mean ± s.d. (n ≥ 3). *** p < 0.001.
The specificity of drug uptake was confirmed in 3D HeLa spheroids and folic acid competitive binding to the cells was performed by exposing the HeLa spheroids with the varying concentrations of plain LP and F/B-LP labeling Cou6 in the presence and absence of excess amount of folic acid in the medium. The fluorescent images of concentrations-dependent uptake of plain LP and F/B-LP labeling Cou6 were expressed in Fig. 4d. After cell lysis, the total Cou6 uptake was measured and quantified as mean fluorescence intensity as shown in Fig. 4e. We demonstrated that at a concentration of 50 ng/mL Cou6, F/B-LP tremendously increased the uptake of Cou6 compared to plain LP in the absence of extra amount of folic acid. Whereas, there was no significant difference in Cou6 uptake between plain LP and F/B-LP in the presence of the extra amount of folic acid duo to the competitive uptake of folic acid. The results suggested folate-conjugated liposomes could enhance drug uptake specifically.
Adhesion of F/B-LP under dynamic flow condition
According to the gold of this study to enhance the specificity of MNZ delivery to cancer cells, the specificity of F/B-LP adhesion to the high FR/BR-expressing cells was investigated by using a typical parallel-plate flow chamber as illustrated in Fig. 5a. This assay is the simple top bench approach which can mimic the shear stress in the blood vessel surrounding tumor tissue and can visualize the particle binding in real time under a fluorescence microscope27,51. The obtained result is shown in Fig. 5b, the tremendous difference in binding efficacy between F/B-LP and plain LP was found in HeLa cells, with binding efficacies of 33.26 ± 4.26 and 12.14 ± 2.78 particles/mm2 for F/B-LP and plain LP, respectively. On the other hand, the adhesion of liposomes in HDFa cells was scarcely observed. These findings show that incorporating cationic lipids into liposomes enhances their attachment to the negatively charged phosphatidylserine in the outer membrane of cancer cells compared to normal cells. Additionally, dual folate and biotin targeting significantly increase the binding of liposomes to cancer cells by enhancing adhesive interactions with folate and biotin receptors.

The Binding efficiency of plain LP (grey bars) and F/B-decorated liposomes (black bars) toward cancer (HeLa) cells expressing high levels of FR/BR and normal (HDFa) cells under flow conditions at wall shear rate of 100 s−1. (a) Schematic illustration of adhesion test of liposomes using parallel-plate flow chamber assay. (b) Quantification of the average numbers of adherent particles on HeLa and HDFa cell surfaces. Numbers of bound particles were counted from more than 15 flames of fluorescent images of independent samples. Data are represented as mean ± s.e.m. (n ≥ 3). *** p < 0.001.
Anti-migration of F/B-LP-MNZ in HeLa cells
Migration is a crucial aspect of cancer progression and metastasis, where cancer cells spread from the primary tumor site to other parts of the body. Thus, this experiment aimed to evaluate the effectiveness of folate/biotin-decorated liposomes loading MNZ in inhibiting the ability of cancer cell migration by observing wound closure. The phase-contrast images of wound closure after incubating with each sample at 0, 24, and 48 h were presented in Fig. 6a. After that, percentages of wound confluence were quantified as shown in Fig. 6b. We found F/B-LP-MNZ significantly slowed the wound closure compared to those of untreated control and empty liposomes groups at 24 and 48 h.

The effect of folate/biotin-decorated liposomes loading MNZ (F/B-LP-MNZ) on inhibiting migration of HeLa cells. (a) Phase-contrast imaging of wound closure at 0, 24, and 48 h. Cells with straight wound lines were cultured in DMEM-low glucose supplemented with 10% FBS in the presence and absence of liposomes (equal to 2 µM MNZ of loaded drug) for 48 h. Wound closure was monitored under an inverted microscope at 4 × magnification and phase-contrast images of cell migration were taken at indicated times. Scale bars are 500 µm. (b) Quantitative analysis of relative cell migration at indicated times. The empty area at each time point was quantified using ImageJ software. The effect of MNZ on the expression of genes involving anti-metastasis and apoptosis processes was analyzed using qRT-PCR: MMP9 (c), BAX (d), and BCL-xL (e). Data are represented as mean ± SD (n ≥ 3). *p < 0.001, **p < 0.001, and ***p < 0.001.
Epithelial-mesenchymal transition (EMT) is a vital biological process for cancer progression and metastasis. During EMT, the zinc-finger-transcription factors such as Snail, Slug and Twist, and matrix metalloproteinases (MMPs) will cooperate to modulate the EMT process52,53. To further investigate the mechanism of MNZ in suppressing proliferation and metastasis in HeLa cells. The mRNA expressions of MMP9, anti-apoptotic (Bcl-xL), and pro-apoptotic (Bax) Bcl-2 proteins were evaluated using qRT-PCR, as shown in Fig. 6c–e. The result revealed that MMP9 and Bcl-xL were downregulated and Bax was upregulated after being treated with MNZ, indicating that MNZ inhibited cancer progression and metastasis by suppressing EMT and inducing apoptotic processes.
Suppression of cell adhesion of F/B-LP-MNZ in HeLa cells
Cell adhesion is a fundamental process in cancer metastasis. Herein, the effect of folate/biotin-decorated liposomes loading MNZ on inhibition of the ability of cancer cell adhesion to cell culture plate was observed. The images representing the amount of HeLa cells that were able to adhere after 2 h plating the cells in the presence of varying concentrations of F/B-LP-MNZ were shown in supplementary Fig. S5a. LP-Blank was used as a negative control. The quantified results of relative percentage of cell adhesion were compared to the untreated control as shown in supplementary Fig. S5b. The result revealed that F/B-LP-MNZ significantly reduced the adhesion of HeLa cells in a concentration-dependent manner compared to those of concentrations of LP-Blank. According to previous studies, suppression of cell adhesion by naphthoquinones likely involves multiple mechanisms, including modulation of adhesion molecule expression54, disruption of integrin signaling54, induction of apoptosis54, inhibition of cell migration54, and reduction of ROS levels55. But not in this case, naphthazarin can induce ROS-based mitochondria-mediated apoptosis56. Overall, these results suggested that MNZ plays an important role in suppression of cell adhesion by preventing cells from interacting with the extracellular matrix. However, further research is needed to fully understand the precise mechanisms underlying the effects of MNZ on cell adhesion.
Conclusion
We had successfully synthesized methylnaphthazarin and developed liposomes decorating folate and biotin for delivery of methylnaphthazarin targeting cancer cells. The favorable physicochemical characteristics and stability of the methylnaphthazarin-loaded liposomes suggested their suitability for use as drug carriers. In acidic conditions simulating tumor microenvironment exhibited the preferential release of methylnaphthazarin from liposomes compared to neutral conditions. The decoration of folate and biotin on the surface of liposomes could increase the inhibition of cell viability of HeLa cells in both monolayers and 3D spheroids better than non-modified liposomes. Subsequently, the specificity of drug delivery of folate/biotin-decorated liposomes was investigated in HeLa cells representing cancer cells expressing high levels of folate and biotin receptors. We demonstrated that folate/biotin-decorated liposomes significantly enhanced drug uptake in both monolayers and 3D spheroids of HeLa in comparison to non-decorated liposomes. The study of liposome adhesion under dynamic flow conditions indicated the outstanding capability of folate/biotin-decorated liposomes in promoting the binding to targeted cancer cells. Taken together with anti-metastasis properties, these results highlight the interesting trends in further studies of tumor suppression in vivo and clinical of folate/biotin-decorated liposomes encapsulated methylnaphthazarin as a potential cancer therapeutic agent.
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and its Supplementary Information files. Should any raw data files be needed in another format they are available from the corresponding author upon reasonable request.
References
-
Emran, T. B. et al. Multidrug resistance in cancer: understanding molecular mechanisms, Immunoprevention and therapeutic approaches. Front. Oncol. 12, 891652. https://doi.org/10.3389/fonc.2022.891652 (2022).
Google Scholar
-
Havrilesky, L. J., Garfield, C. F., Barnett, J. C. & Cohn, D. E. Economic impact of paclitaxel shortage in patients with newly diagnosed ovarian cancer. Gynecol. Oncol. 125, 631–634. https://doi.org/10.1016/j.ygyno.2012.03.028 (2012).
Google Scholar
-
Cohen, P. R. Paclitaxel-associated reticulate hyperpigmentation: Report and review of chemotherapy-induced reticulate hyperpigmentation. World J. Clin. Cases 4, 390–400. https://doi.org/10.12998/wjcc.v4.i12.390 (2016).
Google Scholar
-
Chen, Z. et al. Current understanding of passive and active targeting nanomedicines to enhance tumor accumulation. Coord. Chem. Rev. 481, 215051. https://doi.org/10.1016/j.ccr.2023.215051 (2023).
Google Scholar
-
Bazak, R., Houri, M., El Achy, S., Kamel, S. & Refaat, T. Cancer active targeting by nanoparticles: A comprehensive review of literature. J. Cancer Res. Clin. Oncol. 141, 769–784. https://doi.org/10.1007/s00432-014-1767-3 (2015).
Google Scholar
-
Kang, J. H., Jang, W. Y. & Ko, Y. T. The effect of surface charges on the cellular uptake of liposomes investigated by live cell imaging. Pharm. Res. 34, 704–717. https://doi.org/10.1007/s11095-017-2097-3 (2017).
Google Scholar
-
Tomori, Y. et al. Morphological analysis of trafficking and processing of anionic and cationic liposomes in cultured cells. Acta Histochem. Cytochem. 51, 81–92. https://doi.org/10.1267/ahc.17021 (2018).
Google Scholar
-
Pathak, C. et al. Insights of endocytosis signaling in health and disease. Int. J. Mol. Sci. 24, 2971. https://doi.org/10.3390/ijms24032971 (2023).
Google Scholar
-
Akbarzadeh, A. et al. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 8, 102. https://doi.org/10.1186/1556-276X-8-102 (2013).
Google Scholar
-
Nakhaei, P. et al. Liposomes: structure, biomedical applications, and stability parameters with emphasis on cholesterol. Front. Bioeng. Biotechnol. 9, 705886. https://doi.org/10.3389/fbioe.2021.705886 (2021).
Google Scholar
-
Mohamed, M. et al. PEGylated liposomes: Immunological responses. Sci. Technol. Adv. Mater. 20, 710–724. https://doi.org/10.1080/14686996.2019.1627174 (2019).
Google Scholar
-
Anabousi, S. et al. Effect of PEGylation on the stability of liposomes during nebulisation and in lung surfactant. J. Nanosci. Nanotechnol. 6, 3010–3016. https://doi.org/10.1166/jnn.2006.461 (2006).
Google Scholar
-
Veselov, V. V., Nosyrev, A. E., Jicsinszky, L., Alyautdin, R. N. & Cravotto, G. Targeted delivery methods for anticancer drugs. Cancers 14, 622. https://doi.org/10.3390/cancers14030622 (2022).
Google Scholar
-
Antignani, A. et al. Targeting receptors on cancer cells with protein toxins. Biomolecules 10, 1331. https://doi.org/10.3390/biom10091331 (2020).
Google Scholar
-
Wu, X. et al. Targeting effect of betulinic acid liposome modified by hyaluronic acid on hepatoma cells in vitro. J. Pharm. Sci. 111, 3047–3053. https://doi.org/10.1016/j.xphs.2022.06.015 (2022).
Google Scholar
-
Ju, R.-J. et al. Hyaluronic acid modified daunorubicin plus honokiol cationic liposomes for the treatment of breast cancer along with the elimination vasculogenic mimicry channels. J. Drug Target 26, 793–805. https://doi.org/10.1080/1061186X.2018.1428809 (2018).
Google Scholar
-
Zavaleta, C. L., Phillips, W. T., Soundararajan, A. & Goins, B. A. Use of avidin/biotin-liposome system for enhanced peritoneal drug delivery in an ovarian cancer model. Int. J. Pharm. 337, 316–328. https://doi.org/10.1016/j.ijpharm.2007.01.010 (2007).
Google Scholar
-
Huang, M. et al. Biotin and glucose dual-targeting, ligand-modified liposomes promote breast tumor-specific drug delivery. Bioorganic Med. Chem. Lett. 30, 127151. https://doi.org/10.1016/j.bmcl.2020.127151 (2020).
Google Scholar
-
Tong, Y. et al. Dual-targeted cationic liposomes modified with hyaluronic acid and folic acid deliver siRNA Bcl-2 in the treatment of cervical cancer. https://doi.org/10.21203/rs.3.rs-20688/v1 (2020).
-
Handali, S. et al. A novel 5-Fluorouracil targeted delivery to colon cancer using folic acid conjugated liposomes. Biomed. Pharmacother. 108, 1259–1273. https://doi.org/10.1016/j.biopha.2018.09.128 (2018).
Google Scholar
-
Wen, X. et al. Anticancer efficacy of targeted shikonin liposomes modified with RGD in breast cancer cells. Molecules 23, 268. https://doi.org/10.3390/molecules23020268 (2018).
Google Scholar
-
Ji, S. et al. RGD-conjugated albumin nanoparticles as a novel delivery vehicle in pancreatic cancer therapy. Cancer Biol. Ther. 13, 206–215. https://doi.org/10.4161/cbt.13.4.18692 (2012).
Google Scholar
-
Likhitwitayawuid, K., Kaewamatawong, R., Ruangrungsi, N. & Krungkrai, J. Antimalarial naphthoquinones from Nepenthes thorelii. Planta Med. 64, 237–241. https://doi.org/10.1055/s-2006-957417 (1998).
Google Scholar
-
Budzianowski, J. 2-Methylnaphthazarin 5-O-glucoside from the methanol extracts of in vitro cultures of Drosera species. Phytochemistry 44, 75–77. https://doi.org/10.1016/S0031-9422(96)00520-1 (1997).
Google Scholar
-
Zhang, J. et al. Synthesis of naphthazarin derivatives and identification of novel thioredoxin reductase inhibitor as potential anticancer agent. Eur. J. Med. Chem. 140, 435–447. https://doi.org/10.1016/j.ejmech.2017.09.027 (2017).
Google Scholar
-
Wongkhieo, S. et al. Liposomal thiostrepton formulation and its effect on breast cancer growth inhibition. J. Pharm. Sci. 110, 2508–2516. https://doi.org/10.1016/j.xphs.2021.01.018 (2021).
Google Scholar
-
Namdee, K. et al. Cell-based assay for characterizing cell adhesion properties of active targeted nanoparticles under static and flow condition using an integrated flow chamber. J. Drug Deliv. Sci. Technol. 45, 296–302. https://doi.org/10.1016/j.jddst.2018.03.018 (2018).
Google Scholar
-
Qi, C. et al. Co-delivery of curcumin and capsaicin by dual-targeting liposomes for inhibition of aHSC-induced drug resistance and metastasis. ACS Appl. Mater. Interfaces 13, 16019–16035. https://doi.org/10.1021/acsami.0c23137 (2021).
Google Scholar
-
Zhang, Z. & Yao, J. Preparation of irinotecan-loaded folate-targeted liposome for tumor targeting delivery and its antitumor activity. AAPS PharmSciTech 13, 802–810. https://doi.org/10.1208/s12249-012-9776-5 (2012).
Google Scholar
-
Zhao, W., Zhuang, S. & Qi, X. R. Comparative study of the in vitro and in vivo characteristics of cationic and neutral liposomes. Int. J. Nanomed. 6, 3087–3098. https://doi.org/10.2147/ijn.S25399 (2011).
Google Scholar
-
Miatmoko, A., Asmoro, F. H., Azhari, A. A., Rosita, N. & Huang, C.-S. The effect of 1,2-dioleoyl-3-trimethylammonium propane (DOTAP) Addition on the physical characteristics of β-ionone liposomes. Sci. Rep. 13, 4324. https://doi.org/10.1038/s41598-023-31560-5 (2023).
Google Scholar
-
Krasnici, S. et al. Effect of the surface charge of liposomes on their uptake by angiogenic tumor vessels. Int. J. Cancer 105, 561–567. https://doi.org/10.1002/ijc.11108 (2003).
Google Scholar
-
Guimarães, D., Cavaco-Paulo, A. & Nogueira, E. Design of liposomes as drug delivery system for therapeutic applications. Int. J. Pharm. 601, 120571. https://doi.org/10.1016/j.ijpharm.2021.120571 (2021).
Google Scholar
-
Hanafy, M. S. et al. Effect of the amount of cationic lipid used to complex siRNA on the cytotoxicity and proinflammatory activity of siRNA-solid lipid nanoparticles. Int. J. Pharm. X 6, 100197. https://doi.org/10.1016/j.ijpx.2023.100197 (2023).
Google Scholar
-
Hernández-Caselles, T., Villalaín, J. & Gómez-Fernández, J. C. Stability of liposomes on long term storage. J. Pharm. Pharmacol. 42, 397–400. https://doi.org/10.1111/j.2042-7158.1990.tb06578.x (1990).
Google Scholar
-
Tsubone, T. M., Baptista, M. S. & Itri, R. Understanding membrane remodelling initiated by photosensitized lipid oxidation. Biophys. Chem. 254, 106263. https://doi.org/10.1016/j.bpc.2019.106263 (2019).
Google Scholar
-
Justus, C. R., Dong, L. & Yang, L. V. Acidic tumor microenvironment and pH-sensing G protein-coupled receptors. Front. Physiol. 4, 354. https://doi.org/10.3389/fphys.2013.00354 (2013).
Google Scholar
-
Liu, J. et al. pH-sensitive nano-systems for drug delivery in cancer therapy. Biotechnol. Adv. 32, 693–710. https://doi.org/10.1016/j.biotechadv.2013.11.009 (2014).
Google Scholar
-
Almurshedi, A. S. et al. A novel pH-sensitive liposome to trigger delivery of afatinib to cancer cells: Impact on lung cancer therapy. J. Mol. Liq. 259, 154–166. https://doi.org/10.1016/j.molliq.2018.03.024 (2018).
Google Scholar
-
Hosoya, T. et al. Chromophores in cellulosics, XVIII. Degradation of the cellulosic key chromophore 5,8-dihydroxy-[1,4]-naphthoquinone under conditions of chlorine dioxide pulp bleaching: a combined experimental and theoretical study. Cellulose 25, 4941–4954. https://doi.org/10.1007/s10570-018-1912-2 (2018).
Google Scholar
-
Linares, M., de Bertorello, M. A. M. & Longhi, M. Solubilization of naphthoquinones by complexation with hydroxypropyl-β-cyclodextrin. Int. J. Pharm. 159, 13–18. https://doi.org/10.1016/S0378-5173(97)00269-X (1997).
Google Scholar
-
Lide, D. R. & Milne, G. W. A. Handbook of Data on Organic Compounds Vol. 1 (CRC Press Inc, 1994).
-
Yu, Y., Wang, J., Kaul, S. C., Wadhwa, R. & Miyako, E. Folic acid receptor-mediated targeting enhances the cytotoxicity, efficacy, and selectivity of Withania somnifera leaf extract: In vitro and in vivo evidence. Front. Oncol. https://doi.org/10.3389/fonc.2019.00602 (2019).
Google Scholar
-
Cheung, A. et al. Targeting folate receptor alpha for cancer treatment. Oncotarget 7, 52553–52574. https://doi.org/10.18632/oncotarget.9651 (2016).
Google Scholar
-
Ren, W. et al. Recent development of biotin conjugation in biological imaging, sensing, and target delivery. Chem. Commun. (Camb) 51, 10403–10418. https://doi.org/10.1039/c5cc03075g (2015).
Google Scholar
-
Sangha, V., Hoque, M. T., Henderson, J. T. & Bendayan, R. Novel localization of folate transport systems in the murine central nervous system. Fluids Barriers CNS 19, 92. https://doi.org/10.1186/s12987-022-00391-3 (2022).
Google Scholar
-
Fam, K. T., Collot, M. & Klymchenko, A. S. Probing biotin receptors in cancer cells with rationally designed fluorogenic squaraine dimers. Chem. Sci. 11, 8240–8248. https://doi.org/10.1039/d0sc01973a (2020).
Google Scholar
-
Oh, N. & Park, J. H. Endocytosis and exocytosis of nanoparticles in mammalian cells. Int. J. Nanomed. 9, 51–63. https://doi.org/10.2147/ijn.S26592 (2014).
Google Scholar
-
Banerjee, A., Qi, J., Gogoi, R., Wong, J. & Mitragotri, S. Role of nanoparticle size, shape and surface chemistry in oral drug delivery. J. Control Release 238, 176–185. https://doi.org/10.1016/j.jconrel.2016.07.051 (2016).
Google Scholar
-
Chiangjong, W., Chutipongtanate, S. & Hongeng, S. Anticancer peptide: Physicochemical property, functional aspect and trend in clinical application (Review). Int. J. Oncol. 57, 678–696. https://doi.org/10.3892/ijo.2020.5099 (2020).
Google Scholar
-
Sakariassen, K. S., Orning, L. & Turitto, V. T. The impact of blood shear rate on arterial thrombus formation. Future Sci. OA 1, Fso30. https://doi.org/10.4155/fso.15.28 (2015).
Google Scholar
-
Lin, C.-Y. et al. Matrix metalloproteinase-9 cooperates with transcription factor Snail to induce epithelial–mesenchymal transition. Cancer Sci 102, 815–827. https://doi.org/10.1111/j.1349-7006.2011.01861.x (2011).
Google Scholar
-
Agraval, H. & Yadav, U. C. S. MMP-2 and MMP-9 mediate cigarette smoke extract-induced epithelial-mesenchymal transition in airway epithelial cells via EGFR/Akt/GSK3β/β-catenin pathway: Amelioration by fisetin. Chem. Biol. Interact. 314, 108846. https://doi.org/10.1016/j.cbi.2019.108846 (2019).
Google Scholar
-
Wang, H. et al. Shikonin attenuates lung cancer cell adhesion to extracellular matrix and metastasis by inhibiting integrin β1 expression and the ERK1/2 signaling pathway. Toxicology 308, 104–112. https://doi.org/10.1016/j.tox.2013.03.015 (2013).
Google Scholar
-
San Martín, A. & Griendling, K. K. Redox control of vascular smooth muscle migration. Antioxid Redox Signal 12, 625–640. https://doi.org/10.1089/ars.2009.2852 (2010).
Google Scholar
-
Liang, W. et al. Shikonin induces ROS-based mitochondria-mediated apoptosis in colon cancer. Oncotarget 8, 109094–109106. https://doi.org/10.18632/oncotarget.22618 (2017).
Google Scholar
Acknowledgements
This research was supported by the Chulalongkorn University–NSTDA Doctoral Scholarship and the 90th Anniversary of Chulalongkorn University Scholarship under the Ratchadapisek Somphot Endowment Fund. Furthermore, the researchers would like to express our gratitude to Department of Chemistry, Faculty of Science, Chulalongkorn University, Thailand, and National Nanotechnology Center (NANOTEC), National Science and Technology Development Agency (NSTDA), Thailand for providing the facilities, equipment, and Fundamental Fund 2024 (P2351510).
Author information
Authors and Affiliations
Contributions
P.M. designed the research, performed all experiments, analyzed the data, and prepared and revised the manuscript. W.C. and M.K. were supervisors, provided valuable guidance and funding, and revised the paper. All authors have read and approved the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Information.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
Reprints and permissions
About this article
Cite this article
Mikled, P., Chavasiri, W. & Khongkow, M. Dual folate/biotin-decorated liposomes mediated delivery of methylnaphthazarin for anti-cancer activity.
Sci Rep 14, 21796 (2024). https://doi.org/10.1038/s41598-024-72532-7
-
Received: 20 June 2024
-
Accepted: 09 September 2024
-
Published: 18 September 2024
-
DOI: https://doi.org/10.1038/s41598-024-72532-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.