Abstract
Background/Objectives
Consumers of home parenteral nutrition (HPN) are susceptible to dysglycemia. The aim was to characterize 24-h glucose profiles of HPN consumers using continuous glucose monitors (CGM) and to identify factors that influence glucose.
Subjects/Methods
Glucose profiles of 20 adults with short bowel syndrome (SBS) without diabetes were assessed using the Freestyle Libre Pro CGM. Measures included mean 24-h glucose, coefficient of variation (%), % time in range (TIR 70–140 mg/dL), among others. HPN parameters and lifestyle behaviors were obtained from self-reports and validated surveys. Linear mixed-effects models were used to test associations with glycemic measures adjusted for age, sex, and BMI. Significance was considered at P < 0.05.
Results
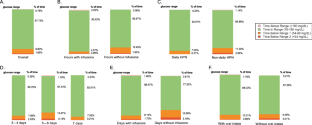
Participants (77% female, age = 52 years, BMI = 21.4 kg/m², 95% white) had a 24-h mean and CV for glucose of 94.69 (8.96) mg/dL and 20.27%, respectively, and a mean TIR of 87.73%. Among non-daily HPN-dependent patients, the mean glucose and TIR were higher on days receiving HPN. Tapering HPN was associated with −6.882 (95% confidence interval = −12.436, −1.329) % lower CV, and higher HPN dextrose content per gram was associated with 0.039 (95% confidence interval = 0.008, 0.07) % higher CV. Smoking, more depressive symptoms, and higher insomnia severity showed associations with glucose levels and variability.
Conclusions
Metabolically stable HPN adult consumers have 24-h glucose measures comparable to healthy adults yet are notable for more time spent below range. The glucose profiles are influenced by HPN parameters such as tapering and dextrose and behaviors including smoking, depressive symptoms, and insomnia.
This is a preview of subscription content, access via your institution
Access options
/* style specs end */
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
/* style specs start */
style {
display: none !important;
}
.LiveAreaSection * {
align-content: stretch;
align-items: stretch;
align-self: auto;
animation-delay: 0s;
animation-direction: normal;
animation-duration: 0s;
animation-fill-mode: none;
animation-iteration-count: 1;
animation-name: none;
animation-play-state: running;
animation-timing-function: ease;
azimuth: center;
backface-visibility: visible;
background-attachment: scroll;
background-blend-mode: normal;
background-clip: borderBox;
background-color: transparent;
background-image: none;
background-origin: paddingBox;
background-position: 0 0;
background-repeat: repeat;
background-size: auto auto;
block-size: auto;
border-block-end-color: currentcolor;
border-block-end-style: none;
border-block-end-width: medium;
border-block-start-color: currentcolor;
border-block-start-style: none;
border-block-start-width: medium;
border-bottom-color: currentcolor;
border-bottom-left-radius: 0;
border-bottom-right-radius: 0;
border-bottom-style: none;
border-bottom-width: medium;
border-collapse: separate;
border-image-outset: 0s;
border-image-repeat: stretch;
border-image-slice: 100%;
border-image-source: none;
border-image-width: 1;
border-inline-end-color: currentcolor;
border-inline-end-style: none;
border-inline-end-width: medium;
border-inline-start-color: currentcolor;
border-inline-start-style: none;
border-inline-start-width: medium;
border-left-color: currentcolor;
border-left-style: none;
border-left-width: medium;
border-right-color: currentcolor;
border-right-style: none;
border-right-width: medium;
border-spacing: 0;
border-top-color: currentcolor;
border-top-left-radius: 0;
border-top-right-radius: 0;
border-top-style: none;
border-top-width: medium;
bottom: auto;
box-decoration-break: slice;
box-shadow: none;
box-sizing: border-box;
break-after: auto;
break-before: auto;
break-inside: auto;
caption-side: top;
caret-color: auto;
clear: none;
clip: auto;
clip-path: none;
color: initial;
column-count: auto;
column-fill: balance;
column-gap: normal;
column-rule-color: currentcolor;
column-rule-style: none;
column-rule-width: medium;
column-span: none;
column-width: auto;
content: normal;
counter-increment: none;
counter-reset: none;
cursor: auto;
display: inline;
empty-cells: show;
filter: none;
flex-basis: auto;
flex-direction: row;
flex-grow: 0;
flex-shrink: 1;
flex-wrap: nowrap;
float: none;
font-family: initial;
font-feature-settings: normal;
font-kerning: auto;
font-language-override: normal;
font-size: medium;
font-size-adjust: none;
font-stretch: normal;
font-style: normal;
font-synthesis: weight style;
font-variant: normal;
font-variant-alternates: normal;
font-variant-caps: normal;
font-variant-east-asian: normal;
font-variant-ligatures: normal;
font-variant-numeric: normal;
font-variant-position: normal;
font-weight: 400;
grid-auto-columns: auto;
grid-auto-flow: row;
grid-auto-rows: auto;
grid-column-end: auto;
grid-column-gap: 0;
grid-column-start: auto;
grid-row-end: auto;
grid-row-gap: 0;
grid-row-start: auto;
grid-template-areas: none;
grid-template-columns: none;
grid-template-rows: none;
height: auto;
hyphens: manual;
image-orientation: 0deg;
image-rendering: auto;
image-resolution: 1dppx;
ime-mode: auto;
inline-size: auto;
isolation: auto;
justify-content: flexStart;
left: auto;
letter-spacing: normal;
line-break: auto;
line-height: normal;
list-style-image: none;
list-style-position: outside;
list-style-type: disc;
margin-block-end: 0;
margin-block-start: 0;
margin-bottom: 0;
margin-inline-end: 0;
margin-inline-start: 0;
margin-left: 0;
margin-right: 0;
margin-top: 0;
mask-clip: borderBox;
mask-composite: add;
mask-image: none;
mask-mode: matchSource;
mask-origin: borderBox;
mask-position: 0 0;
mask-repeat: repeat;
mask-size: auto;
mask-type: luminance;
max-height: none;
max-width: none;
min-block-size: 0;
min-height: 0;
min-inline-size: 0;
min-width: 0;
mix-blend-mode: normal;
object-fit: fill;
object-position: 50% 50%;
offset-block-end: auto;
offset-block-start: auto;
offset-inline-end: auto;
offset-inline-start: auto;
opacity: 1;
order: 0;
orphans: 2;
outline-color: initial;
outline-offset: 0;
outline-style: none;
outline-width: medium;
overflow: visible;
overflow-wrap: normal;
overflow-x: visible;
overflow-y: visible;
padding-block-end: 0;
padding-block-start: 0;
padding-bottom: 0;
padding-inline-end: 0;
padding-inline-start: 0;
padding-left: 0;
padding-right: 0;
padding-top: 0;
page-break-after: auto;
page-break-before: auto;
page-break-inside: auto;
perspective: none;
perspective-origin: 50% 50%;
pointer-events: auto;
position: static;
quotes: initial;
resize: none;
right: auto;
ruby-align: spaceAround;
ruby-merge: separate;
ruby-position: over;
scroll-behavior: auto;
scroll-snap-coordinate: none;
scroll-snap-destination: 0 0;
scroll-snap-points-x: none;
scroll-snap-points-y: none;
scroll-snap-type: none;
shape-image-threshold: 0;
shape-margin: 0;
shape-outside: none;
tab-size: 8;
table-layout: auto;
text-align: initial;
text-align-last: auto;
text-combine-upright: none;
text-decoration-color: currentcolor;
text-decoration-line: none;
text-decoration-style: solid;
text-emphasis-color: currentcolor;
text-emphasis-position: over right;
text-emphasis-style: none;
text-indent: 0;
text-justify: auto;
text-orientation: mixed;
text-overflow: clip;
text-rendering: auto;
text-shadow: none;
text-transform: none;
text-underline-position: auto;
top: auto;
touch-action: auto;
transform: none;
transform-box: borderBox;
transform-origin: 50% 50%0;
transform-style: flat;
transition-delay: 0s;
transition-duration: 0s;
transition-property: all;
transition-timing-function: ease;
vertical-align: baseline;
visibility: visible;
white-space: normal;
widows: 2;
width: auto;
will-change: auto;
word-break: normal;
word-spacing: normal;
word-wrap: normal;
writing-mode: horizontalTb;
z-index: auto;
-webkit-appearance: none;
-moz-appearance: none;
-ms-appearance: none;
appearance: none;
margin: 0;
}
.LiveAreaSection {
width: 100%;
}
.LiveAreaSection .login-option-buybox {
display: block;
width: 100%;
font-size: 17px;
line-height: 30px;
color: #222;
padding-top: 30px;
font-family: Harding, Palatino, serif;
}
.LiveAreaSection .additional-access-options {
display: block;
font-weight: 700;
font-size: 17px;
line-height: 30px;
color: #222;
font-family: Harding, Palatino, serif;
}
.LiveAreaSection .additional-login > li:not(:first-child)::before {
transform: translateY(-50%);
content: “”;
height: 1rem;
position: absolute;
top: 50%;
left: 0;
border-left: 2px solid #999;
}
.LiveAreaSection .additional-login > li:not(:first-child) {
padding-left: 10px;
}
.LiveAreaSection .additional-login > li {
display: inline-block;
position: relative;
vertical-align: middle;
padding-right: 10px;
}
.BuyBoxSection {
display: flex;
flex-wrap: wrap;
flex: 1;
flex-direction: row-reverse;
margin: -30px -15px 0;
}
.BuyBoxSection .box-inner {
width: 100%;
height: 100%;
padding: 30px 5px;
display: flex;
flex-direction: column;
justify-content: space-between;
}
.BuyBoxSection p {
margin: 0;
}
.BuyBoxSection .readcube-buybox {
background-color: #f3f3f3;
flex-shrink: 1;
flex-grow: 1;
flex-basis: 255px;
background-clip: content-box;
padding: 0 15px;
margin-top: 30px;
}
.BuyBoxSection .subscribe-buybox {
background-color: #f3f3f3;
flex-shrink: 1;
flex-grow: 4;
flex-basis: 300px;
background-clip: content-box;
padding: 0 15px;
margin-top: 30px;
}
.BuyBoxSection .subscribe-buybox-nature-plus {
background-color: #f3f3f3;
flex-shrink: 1;
flex-grow: 4;
flex-basis: 100%;
background-clip: content-box;
padding: 0 15px;
margin-top: 30px;
}
.BuyBoxSection .title-readcube,
.BuyBoxSection .title-buybox {
display: block;
margin: 0;
margin-right: 10%;
margin-left: 10%;
font-size: 24px;
line-height: 32px;
color: #222;
text-align: center;
font-family: Harding, Palatino, serif;
}
.BuyBoxSection .title-asia-buybox {
display: block;
margin: 0;
margin-right: 5%;
margin-left: 5%;
font-size: 24px;
line-height: 32px;
color: #222;
text-align: center;
font-family: Harding, Palatino, serif;
}
.BuyBoxSection .asia-link,
.Link-328123652,
.Link-2926870917,
.Link-2291679238,
.Link-595459207 {
color: #069;
cursor: pointer;
text-decoration: none;
font-size: 1.05em;
font-family: -apple-system, BlinkMacSystemFont, “Segoe UI”, Roboto,
Oxygen-Sans, Ubuntu, Cantarell, “Helvetica Neue”, sans-serif;
line-height: 1.05em6;
}
.BuyBoxSection .access-readcube {
display: block;
margin: 0;
margin-right: 10%;
margin-left: 10%;
font-size: 14px;
color: #222;
padding-top: 10px;
text-align: center;
font-family: -apple-system, BlinkMacSystemFont, “Segoe UI”, Roboto,
Oxygen-Sans, Ubuntu, Cantarell, “Helvetica Neue”, sans-serif;
line-height: 20px;
}
.BuyBoxSection ul {
margin: 0;
}
.BuyBoxSection .link-usp {
display: list-item;
margin: 0;
margin-left: 20px;
padding-top: 6px;
list-style-position: inside;
}
.BuyBoxSection .link-usp span {
font-size: 14px;
color: #222;
font-family: -apple-system, BlinkMacSystemFont, “Segoe UI”, Roboto,
Oxygen-Sans, Ubuntu, Cantarell, “Helvetica Neue”, sans-serif;
line-height: 20px;
}
.BuyBoxSection .access-asia-buybox {
display: block;
margin: 0;
margin-right: 5%;
margin-left: 5%;
font-size: 14px;
color: #222;
padding-top: 10px;
text-align: center;
font-family: -apple-system, BlinkMacSystemFont, “Segoe UI”, Roboto,
Oxygen-Sans, Ubuntu, Cantarell, “Helvetica Neue”, sans-serif;
line-height: 20px;
}
.BuyBoxSection .access-buybox {
display: block;
margin: 0;
margin-right: 10%;
margin-left: 10%;
font-size: 14px;
color: #222;
opacity: 0.8px;
padding-top: 10px;
text-align: center;
font-family: -apple-system, BlinkMacSystemFont, “Segoe UI”, Roboto,
Oxygen-Sans, Ubuntu, Cantarell, “Helvetica Neue”, sans-serif;
line-height: 20px;
}
.BuyBoxSection .price-buybox {
display: block;
font-size: 30px;
color: #222;
font-family: -apple-system, BlinkMacSystemFont, “Segoe UI”, Roboto,
Oxygen-Sans, Ubuntu, Cantarell, “Helvetica Neue”, sans-serif;
padding-top: 30px;
text-align: center;
}
.BuyBoxSection .price-buybox-to {
display: block;
font-size: 30px;
color: #222;
font-family: -apple-system, BlinkMacSystemFont, “Segoe UI”, Roboto,
Oxygen-Sans, Ubuntu, Cantarell, “Helvetica Neue”, sans-serif;
text-align: center;
}
.BuyBoxSection .price-info-text {
font-size: 16px;
padding-right: 10px;
color: #222;
font-family: -apple-system, BlinkMacSystemFont, “Segoe UI”, Roboto,
Oxygen-Sans, Ubuntu, Cantarell, “Helvetica Neue”, sans-serif;
}
.BuyBoxSection .price-value {
font-size: 30px;
font-family: -apple-system, BlinkMacSystemFont, “Segoe UI”, Roboto,
Oxygen-Sans, Ubuntu, Cantarell, “Helvetica Neue”, sans-serif;
}
.BuyBoxSection .price-per-period {
font-family: -apple-system, BlinkMacSystemFont, “Segoe UI”, Roboto,
Oxygen-Sans, Ubuntu, Cantarell, “Helvetica Neue”, sans-serif;
}
.BuyBoxSection .price-from {
font-size: 14px;
padding-right: 10px;
color: #222;
font-family: -apple-system, BlinkMacSystemFont, “Segoe UI”, Roboto,
Oxygen-Sans, Ubuntu, Cantarell, “Helvetica Neue”, sans-serif;
line-height: 20px;
}
.BuyBoxSection .issue-buybox {
display: block;
font-size: 13px;
text-align: center;
color: #222;
font-family: -apple-system, BlinkMacSystemFont, “Segoe UI”, Roboto,
Oxygen-Sans, Ubuntu, Cantarell, “Helvetica Neue”, sans-serif;
line-height: 19px;
}
.BuyBoxSection .no-price-buybox {
display: block;
font-size: 13px;
line-height: 18px;
text-align: center;
padding-right: 10%;
padding-left: 10%;
padding-bottom: 20px;
padding-top: 30px;
color: #222;
font-family: -apple-system, BlinkMacSystemFont, “Segoe UI”, Roboto,
Oxygen-Sans, Ubuntu, Cantarell, “Helvetica Neue”, sans-serif;
}
.BuyBoxSection .vat-buybox {
display: block;
margin-top: 5px;
margin-right: 20%;
margin-left: 20%;
font-size: 11px;
color: #222;
padding-top: 10px;
padding-bottom: 15px;
text-align: center;
font-family: -apple-system, BlinkMacSystemFont, “Segoe UI”, Roboto,
Oxygen-Sans, Ubuntu, Cantarell, “Helvetica Neue”, sans-serif;
line-height: 17px;
}
.BuyBoxSection .tax-buybox {
display: block;
width: 100%;
color: #222;
padding: 20px 16px;
text-align: center;
font-family: -apple-system, BlinkMacSystemFont, “Segoe UI”, Roboto,
Oxygen-Sans, Ubuntu, Cantarell, “Helvetica Neue”, sans-serif;
line-height: NaNpx;
}
.BuyBoxSection .button-container {
display: flex;
padding-right: 20px;
padding-left: 20px;
justify-content: center;
}
.BuyBoxSection .button-container > * {
flex: 1px;
}
.BuyBoxSection .button-container > a:hover,
.Button-505204839:hover,
.Button-1078489254:hover,
.Button-2737859108:hover {
text-decoration: none;
}
.BuyBoxSection .btn-secondary {
background: #fff;
}
.BuyBoxSection .button-asia {
background: #069;
border: 1px solid #069;
border-radius: 0;
cursor: pointer;
display: block;
padding: 9px;
outline: 0;
text-align: center;
text-decoration: none;
min-width: 80px;
margin-top: 75px;
}
.BuyBoxSection .button-label-asia,
.ButtonLabel-3869432492,
.ButtonLabel-3296148077,
.ButtonLabel-1636778223 {
display: block;
color: #fff;
font-size: 17px;
line-height: 20px;
font-family: -apple-system, BlinkMacSystemFont, “Segoe UI”, Roboto,
Oxygen-Sans, Ubuntu, Cantarell, “Helvetica Neue”, sans-serif;
text-align: center;
text-decoration: none;
cursor: pointer;
}
.Button-505204839,
.Button-1078489254,
.Button-2737859108 {
background: #069;
border: 1px solid #069;
border-radius: 0;
cursor: pointer;
display: block;
padding: 9px;
outline: 0;
text-align: center;
text-decoration: none;
min-width: 80px;
max-width: 320px;
margin-top: 20px;
}
.Button-505204839 .btn-secondary-label,
.Button-1078489254 .btn-secondary-label,
.Button-2737859108 .btn-secondary-label {
color: #069;
}
.uList-2102244549 {
list-style: none;
padding: 0;
margin: 0;
}
/* style specs end */

Data availability
Participant-level data are available from the Mass General Brigham Human Research Office/Institutional Review Board at Mass General Brigham for researchers who meet the criteria for access to confidential data.
Code availability
The code described for this study is available upon request pending approval by the corresponding author. Researchers interested in reproducing or extending the analysis can request access by contacting the corresponding author.
References
-
Staun M, Pironi L, Bozzetti F, Baxter J, Forbes A, Joly F, et al. ESPEN Guidelines on Parenteral Nutrition: home parenteral nutrition (HPN) in adult patients. Clin Nutr. 2009;28:467–79.
Google Scholar
-
Pironi L, Arends J, Bozzetti F, Cuerda C, Gillanders L, Jeppesen PB, et al. ESPEN guidelines on chronic intestinal failure in adults. Clin Nutr. 2016;35:247–307.
Google Scholar
-
Dibb M, Teubner A, Theis V, Shaffer J, Lal S. Review article: the management of long-term parenteral nutrition. Aliment Pharm Ther. 2013;37:587–603.
Google Scholar
-
Reber E, Staub K, Schönenberger KA, Stanga A, Leuenberger M, Pichard C, et al. Management of home parenteral nutrition: complications and survival. Ann Nutr Metab. 2021;77:46–55.
Google Scholar
-
Gosmanov AR, Umpierrez GE. Management of hyperglycemia during enteral and parenteral nutrition therapy. Curr Diab Rep. 2013;13:155–62.
Google Scholar
-
Wang P, Sun H, Maitiabula G, Zhang L, Yang J, Zhang Y, et al. Total parenteral nutrition impairs glucose metabolism by modifying the gut microbiome. Nat Metab. 2023;5:331–48.
Google Scholar
-
Dashti HS, Leong A, Mogensen KM, Annambhotla M, Li P, Deng H, et al. Glycemic and sleep effects of daytime compared with those of overnight infusions of home parenteral nutrition in adults with short bowel syndrome: a quasi-experimental pilot trial. Am J Clin Nutr. 2024;119:569–77.
Google Scholar
-
Bering J, DiBaise JK. Home parenteral and enteral nutrition. Nutrients. 2022;14:2558. https://doi.org/10.3390/nu14132558.
-
Edakkanambeth Varayil J, Yadav S, Miles JM, Okano A, Kelly DG, Hurt RT, et al. Hyperglycemia during home parenteral nutrition administration in patients without diabetes. JPEN J Parenter Enter Nutr. 2017;41:672–7.
Google Scholar
-
Barrera R, Poindexter K, Tucker C, Winkler MF, Dashti HS. Amplifying the lived experiences of parenteral nutrition consumers through the thematic analysis of social media posts. Nutr Clin Pract. 2023;39:850–8. https://doi.org/10.1002/ncp.11097.
-
Rodbard D. Continuous glucose monitoring: a review of recent studies demonstrating improved glycemic outcomes. Diabetes Technol Ther. 2017;19:S25–37.
Google Scholar
-
Karter AJ, Parker MM, Moffet HH, Gilliam LK, Dlott R. Association of real-time continuous glucose monitoring with glycemic control and acute metabolic events among patients with insulin-treated diabetes. JAMA. 2021;325:2273–84.
Google Scholar
-
Liang X, Fu Y, Lu S, Shuai M, Miao Z, Gou W, et al. Continuous glucose monitoring-derived glycemic metrics and adverse pregnancy outcomes among women with gestational diabetes: a prospective cohort study. J Clin Endocrinol Metab. 2014;99:4674–82. https://doi.org/10.1016/j.lanwpc.2023.100823.
-
Zhao T, Fu Y, Zhang T, Guo J, Liao Q, Song S, et al. Diabetes management in patients undergoing total pancreatectomy: a single center cohort study. Front Endocrinol. 2023;14:1097139.
Google Scholar
-
Lee S, Lee S, Kim KM, Shin JH. Usefulness of continuous glucose monitoring of blood glucose control in patients with diabetes undergoing hemodialysis: a pilot study. Front Med. 2023;10:1145470.
Google Scholar
-
Chichester S, Rahmoune A, Dashti HS. Home parenteral nutrition, sleep patterns, and depressive symptoms: secondary analysis of cross-sectional data. JPEN J Parenter Enteral Nutr. 2024;48:709–17. https://doi.org/10.1002/jpen.2631.
-
Bailey T, Bode BW, Christiansen MP, Klaff LJ, Alva S. The performance and usability of a factory-calibrated flash glucose monitoring system. Diabetes Technol Ther. 2015;17:787–94.
Google Scholar
-
Denham D. A head-to-head comparison study of the first-day performance of two factory-calibrated CGM systems. J Diabetes Sci Technol. 2020;14:493–5.
Google Scholar
-
Battelino T, Danne T, Bergenstal RM, Amiel SA, Beck R, Biester T, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42:1593–603.
Google Scholar
-
Kroenke K, Strine TW, Spitzer RL, Williams JBW, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. J Affect Disord. 2009;114:163–73.
Google Scholar
-
Ware J Jr, Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–33.
Google Scholar
-
Bastien CH, Vallières A. Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2:297–307.
Google Scholar
-
Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213.
Google Scholar
-
Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5.
Google Scholar
-
Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110.
Google Scholar
-
Cheung NW, Napier B, Zaccaria C, Fletcher JP. Hyperglycemia is associated with adverse outcomes in patients receiving total parenteral nutrition. Diabetes Care. 2005;28:2367–71.
Google Scholar
-
Compher C, Bingham AL, McCall M, Patel J, Rice TW, Braunschweig C, et al. Guidelines for the provision of nutrition support therapy in the adult critically ill patient: the American Society for Parenteral and Enteral Nutrition. JPEN J Parenter Enter Nutr. 2022;46:12–41.
Google Scholar
-
Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group, Fox LA, Beck RW, Xing D. Variation of interstitial glucose measurements assessed by continuous glucose monitors in healthy, nondiabetic individuals. Diabetes Care. 2010;33:1297–9.
Google Scholar
-
Pleva M, Mirtallo JM, Steinberg SM. Hyperglycemic events in non-intensive care unit patients receiving parenteral nutrition. Nutr Clin Pr. 2009;24:626–34.
Google Scholar
-
Shamanna P, Saboo B, Damodharan S, Mohammed J, Mohamed M, Poon T, et al. Reducing HbA1c in type 2 diabetes using digital twin technology-enabled precision nutrition: a retrospective analysis. Diabetes Ther. 2020;11:2703–14.
Google Scholar
-
Cogle SV, Hutchison AM, Mulherin DW. Finding the sweet spot: managing parenteral nutrition-related glycemic complications in hospitalized adults. Nutr Clin Pr. 2023;38:1263–72.
Google Scholar
-
Jensen MH, Cichosz SL, Hirsch IB, Vestergaard P, Hejlesen O, Seto E. Smoking is associated with increased risk of not achieving glycemic target, increased glycemic variability, and increased risk of hypoglycemia for people with type 1 diabetes. J Diabetes Sci Technol. 2021;15:827–32.
Google Scholar
-
Marchini F, Caputo A, Convertino A, Giuliani C, Bitterman O, Pitocco D, et al. Associations between continuous glucose monitoring (CGM) metrics and psycholinguistic measures: a correlational study. Acta Diabetol. 2024;61:841–5. https://doi.org/10.1007/s00592-024-02244-x.
-
Liu J, Richmond RC, Bowden J, Barry C, Dashti HS, Daghlas I, et al. Assessing the causal role of sleep traits on glycated hemoglobin: a Mendelian randomization study. Diabetes Care. 2022;45:772–81.
Google Scholar
-
Gulati S, Misra A, Tiwari R, Sharma M, Pandey RM, Upadhyay AD, et al. Beneficial effects of premeal almond load on glucose profile on oral glucose tolerance and continuous glucose monitoring: randomized crossover trials in Asian Indians with prediabetes. Eur J Clin Nutr. 2023;77:586–95.
Google Scholar
-
Maiorino MI, Signoriello S, Maio A, Chiodini P, Bellastella G, Scappaticcio L, et al. Effects of continuous glucose monitoring on metrics of glycemic control in diabetes: a systematic review with meta-analysis of randomized controlled trials. Diabetes Care. 2020;43:1146–56.
Google Scholar
-
Perri A, Giordano L, Corsello M, Priolo F, Vento G, Zecca E, et al. Continuous glucose monitoring (CGM) in very low birth weight newborns needing parenteral nutrition: validation and glycemic percentiles. Ital J Pediatr. 2018;44:99.
Google Scholar
-
McCulloch A, Bansiya V, Woodward JM. Addition of insulin to parenteral nutrition for control of hyperglycemia. JPEN J Parenter Enter Nutr. 2018;42:846–54.
Google Scholar
-
Ratliff A, Nishnick A, DeChicco R, Lopez R. Reducing blood glucose testing is safe in patients receiving parenteral nutrition. Nutr Clin Pr. 2018;33:872–8.
Google Scholar
-
Chow KW, Kelly DJ, Gupta R, Miller JD. Use of continuous glucose monitoring to assess parenteral nutrition-induced hyperglycemia in an adult patient with severe COVID-19. JPEN J Parenter Enter Nutr. 2021;45:208–11.
Google Scholar
-
Mirtallo JM, Fabri PJ. Hemoglobin A1C in home parenteral nutrition. JPEN J Parenter Enter Nutr. 1983;7:59–61.
Google Scholar
-
Pironi L, Boeykens K, Bozzetti F, Joly F, Klek S, Lal S, et al. ESPEN guideline on home parenteral nutrition. Clin Nutr. 2020;39:1645–66.
Google Scholar
-
Mundi MS, Mohamed Elfadil O, Hurt RT, Bonnes S, Salonen BR. Management of long-term home parenteral nutrition: Historical perspective, common complications, and patient education and training. JPEN J Parenter Enter Nutr. 2023;47:S24–34.
Google Scholar
-
Tanenbaum ML, Messer LH, Wu CA, Basina M, Buckingham BA, Hessler D, et al. Help when you need it: Perspectives of adults with T1D on the support and training they would have wanted when starting CGM. Diabetes Res Clin Pr. 2021;180:109048.
Google Scholar
-
Patton SR, Clements MA. Psychological reactions associated with continuous glucose monitoring in youth. J Diabetes Sci Technol. 2016;10:656–61.
Google Scholar
-
Basu A, Dube S, Veettil S, Slama M, Kudva YC, Peyser T, et al. Time lag of glucose from intravascular to interstitial compartment in type 1 diabetes. J Diabetes Sci Technol. 2015;9:63–8.
Google Scholar
-
Barua S, A Wierzchowska-McNew R, Deutz NEP, Sabharwal A. Discordance between postprandial plasma glucose measurement and continuous glucose monitoring. Am J Clin Nutr. 2022;116:1059–69.
Google Scholar
Acknowledgements
The Authors thank the research participants who contributed to this research.
Funding
This work was supported by the American Society for Parenteral and Enteral Nutrition (ASPEN) Rhoads Research Foundation. This work was also supported by the National Institute of Health [grant number R00HL153795 to HSD].
Author information
Authors and Affiliations
Contributions
Priyasahi Saravana and Hassan S Dashti contributed to the conception/design of the research; the acquisition, analysis, and interpretation of the data; Meghan Lau contributed to the analysis of the data; Priyasahi Saravana, Meghan Lau, and Hassan S Dashti drafted the manuscript; all authors critically revised the manuscript; and Hassan S Dashti agrees to be fully responsible for ensuring the integrity and accuracy of the work. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This research was performed in accordance with the Declaration of Helsinki and was approved by the Mass General Brigham Institutional Review Board (#2020P003741). Informed consent was obtained from all participants prior to study procedures.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Material
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Cite this article
Saravana, P., Lau, M. & Dashti, H.S. Continuous glucose monitoring in adults with short bowel syndrome receiving overnight infusions of home parenteral nutrition.
Eur J Clin Nutr (2024). https://doi.org/10.1038/s41430-024-01548-z
-
Received: 23 May 2024
-
Revised: 08 November 2024
-
Accepted: 13 November 2024
-
Published: 23 November 2024
-
DOI: https://doi.org/10.1038/s41430-024-01548-z