Aim and hypothesis
This study is aimed to evaluate the overall effectiveness of the care bundles for the management of enteral nutrition in stroke patients by targeting multiple simultaneous interventions. The primary hypothesis is that care bundles for the management of enteral nutrition can reduce feeding intolerance in stroke patients. The secondary hypothesis is that care bundles can reduce complications, improve nutritional status, and result in better recovery for stroke patients.
Trial design and study setting
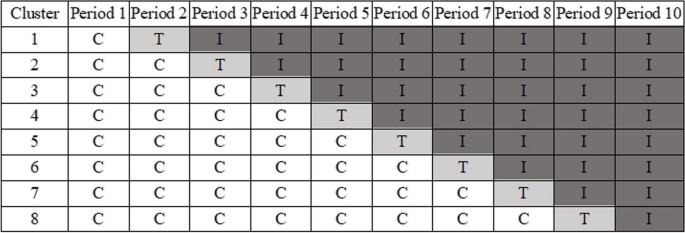
This study is a multi-centre, stepped-wedge, cluster-based randomised controlled trial [17]. As shown in Fig. 1, as the trial progresses, each cluster starts by receiving the control group care and later receives the intervention group care, with the time point at which each cluster shifts care being randomised. The study is designed as a superiority trial and follows the stepped wedge design framework, ultimately achieving a 1:1 allocation ratio of all patients in different clusters between the control and intervention groups. Figure 2 provides a participant timeline of Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) chart [18].

Timeline and randomisation of the stepped wedge cluster randomised trial (C, control period; T, train period; I, intervention period)

SPIRIT chart of the enrolment, interventions, and assessments
The study is to be conducted in eight hospitals (clusters) in the Chinese cities of Chengdu, Deyang, Mianzhu, Luzhou, and Leshan for a duration of 10 periods (each period is about 2 months), which includes a 1-month training period. At the beginning, all hospitals are in the control period and receive the control group care. Starting from the second period, one hospital is randomly selected each month to enter the training period and is switched to the intervention period. By the last period of the study, all the hospitals are in the intervention period and have received the intervention group programme.
Participants
Study site eligibility and recruitment
Eight academic or general hospitals that meet the criteria are selected for inclusion in the study. The hospital criteria are as follows: (i) a tertiary hospital, (ii) a neurosurgery/stroke patient admission ward, (iii) at least 500 stroke patients managed in that ward per year, and (iv) a commitment from hospital administrators to cooperate with the study.
In the recruitment process, we first assess the study capacity, clinical conditions, number of stroke patients admitted, and current status of stroke patient management in each hospital by means of a questionnaire and then contact and invite hospitals with high study capacity that met the hospital criteria and with similar levels of stroke patient management to ensure that the potential interference factors of different centres during the study are as consistent as possible.
Patient eligibility and recruitment
Patients are included in all centres of this study based on the following criteria. The inclusion criteria are as follows: (i) age ≥ 18 years, (ii) diagnosis of stroke, (iii) need for enteral nutrition therapy, and (iv) volunteered to participate in this study. The exclusion criteria are as follows: (i) history of gastrointestinal diseases such as gastroparesis and gastric bleeding and (ii) contraindication of enteral nutrition fluid components. During the trial period, we include all stroke patients who meet the criteria in the neurosurgery department (or stroke patient admission ward) of the hospital.
Sample size
Incidence varies widely due to different definition criteria for feeding intolerance [19]. According to the definition of feeding intolerance in this study, the incidence of feeding intolerance in enteral nutrition stroke patients is 44.1% [10]. We believe that it is clinically meaningful and feasible for the incidence of feeding intolerance in patients to be less than 30% after the intervention, considering clinical experience and published literature [13, 20], according to the sample size calculation formula for stepped wedge cluster randomised trials [21]. We use the Shiny CRT Calculator [22] to calculate the total sample size as 1152 cases with the number of steps = 8, the number of clusters (per step) = 1, preset significance level (α) = 0.05, power (1 − β) = 0.9, and intracluster correlation coefficient (ICC) = 0.01, that is, the sample size of each cluster (hospital) is 144 cases, and 16 samples need to be included in each cluster (hospital) in each cycle. Considering the drop-out of research participants, we plan to include at least 17 samples in each cycle of each hospital (drop-out rate = 6.25%, that is 1/16); thus, the final sample size is 1224.
Randomisation
We will conduct a multi-centre stepped wedge cluster randomised controlled trial in this study. Firstly, eight tertiary general hospitals are selected through open recruitment. In the first period after recruitment, all hospitals begin the study simultaneously, starting with a control period. In the following period, then, a central computer randomisation process will be conducted by a statistics expert who is not part of this study, to determine which hospital will move to the intervention period. The interventions will be part of the care in the hospital until complement of this study. When one period slides on, the interventions will be randomly implemented in a new hospital. Namely, when periods slide on, randomisation process will be performed reduplicatively. In each hospital, there will be staff training that lasts for one month before the formal implementation of interventions. The study is not blind because of the complexity of the design and the intervention.
Interventions
Intervention period
We constructed the care bundles for enteral nutrition management in stroke patients based on the recommendations of the Guidelines and followed the Medical Research Council guidelines on complex interventions [23]. The care bundles include six core measures: (i) establish a nurse-led multidisciplinary stroke enteral nutrition management team, (ii) application of a quality management index system for enteral nutrition in stroke patients, (iii) formation of standardised feeding pathways and modalities (Fig. 3), (iv) establishment of an enteral nutrition monitoring scheme (Fig. 4), (v) provision of individualised enteral nutrition preparation, and (vi) prevention and control of enteral nutrition complications. These measures can operate independently or interdependently—all are designed to standardise the clinical practice of enteral nutrition in stroke patients. Therefore, the care bundles can also be considered a complex intervention [23]. The specific programme elements and implementation strategies are described in the implementation manual of care bundles (Additional file 1). We use a large number of flowcharts and tables to supplement the text content in the manual to ensure the comprehensibility and operability of the care bundles.

The standardised feeding pathways and modalities

The enteral nutrition monitoring scheme
Control period
Patients admitted to the hospital during the control period receive the traditional nutritional measures in the standard of care currently used in the clinic at that hospital. Given the variation in clinical level and management standards across hospitals, there may be some variation in the control period measures across hospitals. The current enteral nutrition nursing in various hospitals includes measures such as management team composition, nutritional risk screening, nutritional status assessment, selection of nutritional preparations, implementation of enteral nutrition, prevention and treatment of complications, and health education. The links involved in these measures are similar to those of the care bundles for enteral nutrition management, but there is a certain gap in the normative and scientific nature of the intervention content. We will investigate the clinical level and current management of stroke patients in each hospital by questionnaire at the time of hospital recruitment, so as to ensure that the control period measures in the final included hospitals are at a similar level.
Implementation processes
The differences between the intervention care and the control care are mainly in the operational aspects of all nursing staff on the ward, where all patients will receive a uniform and homogeneous intervention at the current time, and therefore at a collective level. Given the complexity of the interventions, all interventions are made by trained and qualified nursing staff and are subject to occasional spot checks by the management team during the implementation period to ensure the integrity and standardisation of the implementation of the interventions in each hospital.
Training and assessment
The management team conducts at least 2 weeks of training on the care bundles for enteral nutrition management in stroke patients, starting 1 month before the hospital entered the intervention period. This training is aimed at all nurses in the hospital’s stroke patient ward. The training includes interpretation of the care bundle content and step-by-step explanation of the intervention and clinical practice instruction and includes written material distribution, group teaching, and simulation exercises. After the training, the management team conducts an assessment of the nurses, including theoretical and operational assessments, both of which must be passed before participation in the patient care work, otherwise the training is repeated.
Outcomes
Primary outcome
Feeding intolerance is the primary outcome and is defined as patients with ≥ 2 of the following characteristics during enteral nutrition who are considered feeding intolerant [16, 24]: (i) insufficient enteral intake, as shown by actual enteral nutrition volume less than 2/3 of the planned enteral nutrition volume in 24 h; (ii) patients with gastrointestinal symptoms, as shown by abdominal distension, diarrhoea, vomiting, constipation, regurgitation, aspiration, etc.; (iii) high gastric residual amount, as shown by the monitoring of gastric residual amount > 250 mL or > 50% of enteral intake, 4 h after the last enteral nutrition.
Secondary outcomes
Aspiration pneumonia, rate of achievement of nutrition intake goals, body mass index (BMI), serum prealbumin (PA), serum albumin (ALB), calf circumference, activity of daily living, mortality, and length of hospital stay are the secondary outcomes in this study. Feeding intolerance and rate of achievement of nutrition intake goals will be evaluated daily since participant enrollment, and information about mortality and length of hospital stays will be collected at the discharge. All other outcomes will be evaluated every 7 days since participant enrollment. The definitions, calculation methods, and collection times for each outcome are shown in Table 1.
Process evaluation
The rate of implementation and correctness of intervention period care measured is the focus of this study process monitoring. The management team employ an independent site survey team that uses a combination of periodical verification and random sampling to conduct on-site evaluations at the appropriate hospital at months 1, 3, and 5 of each hospital’s entry into the intervention period as well as at several random time points (a few hospitals will not have evaluations at the third and fifth months due to the study design). Site survey teams conduct monitoring using a process verification form based on the content of the care bundles. The management team regularly summarises the monitoring results, analyses problems in the implementation of the care bundles, explores ways to improve the application of safeguards, and manages the key care operations that could affect patient outcomes.
Data collection and management
The management team will develop clinical report forms (CRFs) based on patient outcomes, and all data will be collected in the form of CRFs and then entered into a unified data management platform through an authorised encrypted account. We establish an independent data collection team at each hospital. Before the official start of the trial, the data collection team at each hospital is trained in a uniform manner, the consistency of data collection is assessed, and data collection is allowed only after the assessment has been passed. The data collection teams collect patient data at the appropriate time through hospital information systems, on-site assessments, and questionnaires and enter the anonymous data that exclude individual characteristics into the data management platform in a timely manner. The platform is set up with reasonable numerical boundaries and logical check items to remind the collector to check the original data in time to correct or reject it when incorrect data entry occurs.
Statistical analyses
Continuous variables collected will be described using mean ± standard deviation (SD) or median and interquartile ranges depending on distribution. Categorical data will be summarised by frequency and percent. A generalised linear mixed model (GLMM) will be utilised to analyse outcomes, with interventions included as fixed effects and hospitals as random effects. The model will be adjusted for demographic factors, Barthel index (BI), and nutritional status at baseline. Additionally, the interaction between time and interventions will be examined and tested for significance. If the interaction term is found to be significant, the simple effects of time and interventions will be evaluated. Otherwise, the model will be reduced to only include main effects. Binary outcomes will be compared between groups using logistic multilevel models. For continuous measurements, normal distribution with identity link function was used. If necessary, log-transformed method will be used to transfer continuous skewed data (i.e. length of stay). The pseudo-likelihood method will be select as the parameter estimation method. Variance component, autocorrelation, and unstructured variance–covariance matrix type are selected as potential candidates used in GLMM and will be finally determined by the goodness of model fit using the Akaike information criterion (AIC). The study will follow the intention-to-treat principle. A per-protocol analysis will also be conducted as the secondary analysis. Notably, no interim analysis is planned in this study. Missing data for individual clinical variables are imputed using clinical imputation rules when appropriate, and the remaining missing data (< 10%) are imputed using the hot-deck imputation method, which is suitable for both continuous and categorical variables. All statistics will be conducted using IBM/SPSS version 23.0. The significance two-sided test level will be set as α = 0.05.
Oversight and monitoring
The chief investigator of the study and the heads of the research centres jointly form the trial steering committee (TSC), which is responsible for overseeing the study progress, directing the research implementation, organising and coordinating the participating centres in conducting the study, and promptly identifying and correcting deviations and errors during execution to ensure that participating centres complete the research on schedule. The TSC holds working meetings every 6 months, and if special matters arise, additional meetings will be convened as needed. The TSC will submit annual reports and regular follow-up review reports to the ethics committee in accordance with its requirements. The management team and sub-centre management teams are established under the TSC, consisting of the key research personnel. These teams are responsible for promoting and reporting on the specific implementation of the study at their respective centres, including identifying potential recruits, taking consent, and organising and training the data collection teams. Any deviation from the protocol will be fully documented. These management team submits progress reports to the TSC every month. In addition, this study has also formed an academic support team composed of experts or professionals in nursing, medicine, nutrition and statistics. The team does not participate in the specific work of project implementation but provides academic support, such as independent trial supervision and implementation of research randomisation. Although this study does not involve the evaluation of new drugs or devices, the independent academic support team will assist the TSC in overseeing and managing the data. They will verify, monitor, and manage the data through the data management platform and collect and check the original (paper) records of the data from each research centre periodically to ensure the accuracy and standardisation of the data.
Adverse event reporting
The care bundles of this study are a collection of clinical measures validated by evidence-based evidence and expert opinion, which we believe to be safe and unlikely to cause adverse events. However, due to the severity of the patients included, there is also a risk of aspiration, refeeding syndrome, catheter migration, catheter-related infections, and death, which are inherently potential risks for the occurrence of adverse events in patients with stroke enteral nutrition. Once an adverse event occurs, it will be handled and tracked according to clinical standards until it is properly resolved or the patient’s condition is stable. All adverse events will be monitored and recorded by the sub-centre management team and will be reported promptly to the TSC, the academic support team, and the ethics committee. If necessary, working meetings will be held to assess the risks and benefits of the study.
Dissemination
The results of the study will be reported in writing to the funder and are planned for publication in a peer-reviewed scientific journal. Additionally, the research content and findings will be disseminated to stakeholders in the field of enteral nutrition for stroke patients through meetings, forums, and other channels.