Abstract
Soluble dietary fiber, notably as an adjunct to early enteral nutrition (EEN), is gaining prominence in clinical therapy. This study evaluates the effect of fructooligosaccharides (FOS), a new soluble dietary fiber, on the prognosis of patients with severe acute pancreatitis (SAP). In a retrospective cohort study at the Third Xiangya Hospital of Central South University from July 2017 to July 2023, 110 SAP patients were analyzed. TPF (enteral nutritional suspension of total protein)-normal and TPF-FOS groups both received standard EEN solutions; the latter additionally received FOS. Outcomes were compared between the groups. The study included 37 patients in the TPF-FOS group and 73 patients in the TPF-normal group. Mortality was 13.50% in the TPF-FOS group and 34.20% in the TPF-normal group (P < 0.05). FOS was identified as an independent protective factor (OR: 0.826, P = 0.041). The TPF-FOS group showed lower rates of intra-abdominal infection and decreased the level of inflammation (P < 0.05). FOS potentially acts as an independent protective factor against death in SAP. Additionally, the supplementation of EEN with FOS may contribute to reducing mortality and improving the prognosis of SAP patients.
Similar content being viewed by others

Kitchen-based diet versus commercial polymeric formulation in acute pancreatitis: a pilot randomized comparative study

Comparative efficacy of peptide-based versus standard polymeric enteral nutrition in ICU patients at high nutritional risk: a multicenter randomized controlled trial

Parenteral fish oil lipid emulsion use in adults: a case series and review from an intestinal failure referral center
Introduction
Severe acute pancreatitis (SAP) is a significant pancreatic inflammatory condition, marked by the abnormal activation of pancreatic enzymes and a widespread inflammatory response. Studies indicated that patients with SAP in-hospital mortality rates can be as high as 30–40%1,2,3. A crucial factor of progression in SAP is the intestinal injury and translocation of gut microbiota, which exacerbate inflammation. This translocation can trigger an inflammatory cascade, intensifying oxidative stress in various organs, potentially leading to systemic inflammatory response syndrome (SIRS) or multiple organ failure (MODS)4,5,6.
In the typical progression of SAP, undernutrition and nutritional risk are commonly observed, with undernutrition being linked to an increased infection risk7. Mederos et al. have demonstrated that early enteral nutrition (EEN) significantly reduces mortality and infection rates in these patients8, highlighting EEN’s critical role in SAP management9. However, SAP often induces gastrointestinal motility disorders and intraperitoneal hypertension, leading to potential interruptions or cessation of EEN10. Prior studies have shown that soluble dietary fiber (SDF) enhances bowel motility in various conditions like constipation, inflammatory bowel disease, irritable bowel syndrome and diabetes11,12,13,14. Moreover, incorporating SDF into EEN has been effective in lowering feeding intolerance symptoms, such as bloating and diarrhea in patients with SAP15.
Fructooligosaccharides (FOS) are novel soluble dietary fibers found in plants like chicory root, bananas, onions, garlic, and leeks. FOS is mainly produced from inulin extracted from chicory, artichoke, yacon, dahlia, or agave roots through enzymatic synthesis or inulin hydrolysis16. Previous research showed that FOS stimulated intestinal motility, modulates gut flora, strengthened the intestinal mucosal barrier, and lowered triglyceride levels17. Oligofructose, a form of FOS, alleviates intestinal stress by upregulating key epithelial genes (ZO-1, LAT1, CAT1, GLUT-2), enhancing barrier integrity, reducing inflammation, and regulating microbial balance18,19,20. However, research on FOS supplementation in EEN for SAP is limited.
The present study was designed to investigate whether FOS supplemented EEN can improve the prognosis in patients with SAP (Fig. 1). We hope this study could provide a new strategy for the clinical management of SAP.

A visual abstract of the study.
Methods
Patients
This retrospective cohort study, approved by the Ethics Committee of the Third Xiangya Hospital of Central South University, enrolled SAP patients from July 2017 to July 2023. This study classified acute pancreatitis (AP) according to the Atlanta 2012 classification. Based on this classification, acute pancreatitis is categorized into three severity levels: Mild Acute Pancreatitis (MAP): No organ failure or local complications. Moderate Acute Pancreatitis (MSAP): Presence of organ failure lasting less than 48 h, or the presence of local complications. SAP: Presence of persistent organ failure or severe local complications (e.g., pancreatic pseudocyst, abscess, etc.). Within 48 h of hospital admission, we confirmed the diagnosis using clinical presentation, imaging studies (such as abdominal CT), and biochemical markers (e.g., serum amylase, lipase), and classified acute pancreatitis according to the Atlanta 2012 classification system. We included only patients diagnosed with SAP in this study.
Inclusion criteria were: (1) SAP diagnosis according to Atlanta 2012 classification; (2) admission within 48 h of onset; (3) EEN via nasogastric tube within 72 h of admission.
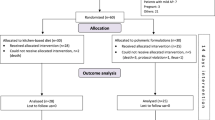
Exclusion criteria included: (1) Transfer from another hospital; (2) Total parenteral nutrition (TPN) received before or during admission; (3) incomplete medical data; (4) age < 18 or pregnancy; (5) Pre-existing chronic diseases (diabetes, cardiovascular diseases, chronic kidney disease, chronic liver disease, chronic respiratory failure etc.) that could confound the outcomes; (6) Previous treatment at an external hospital prior to admission (Fig. 2).

The flow diagram for enrollment of this trial.
Group and sample size
Patients were divided into the TPF (enteral nutritional suspension of total protein)-normal group and the TPF-FOS group based on FOS supplementation. Sample size estimation was based on in-hospital mortality, using pre-phase data from 7 patients receiving FOS-supplemented EEN, where 1 patient died (15% mortality). Estimated mortality for the TPF-normal group was 40%. With α = 0.05, β = 0.2, and a 1:2 ratio (TPF-FOS), the minimum sample size was 32 patients for TPF-FOS and 63 for TPF-normal. Of the 375 patients initially screened, 265 were excluded, leaving 110 eligible patients.
Research design and methods
All patients diagnosed with SAP were transferred to the ICU and received conventional comprehensive treatment based on disease severity. This included gastric acid and pancreatic enzyme inhibition, anti-infection measures, fluid resuscitation, hypoglycemia treatment, analgesia, correction of water and electrolyte imbalances, organ function maintenance, nutritional support, and surgical debridement and drainage as needed. For patients with acute kidney injury unresponsive to fluid resuscitation, continuous renal replacement therapy (CRRT) was administered until kidney function recovered or urine output exceeded 25 ml/h21,22. For patients with respiratory failure unresponsive to conventional oxygen therapy, noninvasive or invasive mechanical ventilation was used until respiratory function was restored. If mean arterial pressure remained < 65 mmHg after fluid resuscitation, vasoactive drugs were administered.
A nasogastric feeding tube (CH/FR15, Fresenius Kabi AG, Germany) was placed in the stomach under gastroscopic guidance within 48 h of admission. If there were no contraindications, EEN was initiated at 72 h23. The TPF-normal group received a 500 ml enteral nutritional suspension of total protein (Fresenius Kabiwari Pharmaceutical Co., China) via a nasogastric tube. The TPF-FOS group received a 500 ml enteral nutritional suspension of total protein combined with FOS (ABBOTT LABORATORIES B.V., Netherlands). Feeding began at 30 ml/h and was adjusted using a pump-assisted system. If tolerated, the rate increased by 20 ml/h every 4 h up to a maximum of 100 ml/h. The target energy requirement was considered met when intake reached 35 kcal/kg/day15. If feeding intolerance occurred, the EEN rate was reduced or suspended. If EEN alone did not meet daily calorie requirements, parenteral nutrition was supplemented24. After being discharged from the ICU, patients typically returned to their primary wards for continued care. The transfer back to the primary ward was based on their clinical improvement and stabilization. Following ICU discharge, patients’ nutritional support was adjusted according to their clinical condition and tolerance. Most patients continued with enteral nutrition (EN) or transitioned to oral nutrition as tolerated. If oral intake was insufficient or if the patient’s condition required additional support, nasogastric tube feeding or parenteral nutrition was continued.
The primary endpoint was in-hospital mortality. Secondary endpoints included total hospital stay, ICU stay length, time to intra-abdominal infection onset, and duration of peristaltic recovery. Abdominal compartment syndrome (ACS) was defined as intra-abdominal pressure > 20 mmHg with new organ dysfunction or failure13. Intra-abdominal pressure was indirectly measured using intravesical pressure21,25. Abdominal infection was defined as a positive culture of pancreatic exudate or stool. Peristaltic recovery time was defined as achieving stool volumes > 500 mL/day. Fever was defined as a temperature > 38 °C.
Data collection and analysis
Demographic characteristics were collected post-admission. Blood biochemical indicators, including blood cell count (WBC), C-reactive protein, procalcitonin (PCT), liver function, renal function, electrolytes, blood glucose, and triglycerides, were assessed within first day of ICU stay (day 1). The Acute Physiology and Chronic Health Evaluation II (APACHE II) score, Sequential Organ Failure Assessment (SOFA) score, Nutrition Risk Screening 2002 (NRS 2002), Modified Marshall score, and Acute Gastrointestinal Injury (AGI) score were also recorded26,27,28. APACHE II evaluates ICU patients’ disease severity, with higher scores indicating greater mortality risk. SOFA monitors organ dysfunction severity, with higher scores correlating to more severe organ failure. NRS 2002 identifies patients needing nutritional support, critical in SAP management. The Modified Marshall score focuses on pancreatitis-related organ dysfunction; higher scores denote greater disease severity. AGI scores categorize gastrointestinal dysfunction extent; higher values signify more severe injury, significant in SAP due to potential gastrointestinal complications. These scores collectively guide clinical management, tailoring treatment strategies based on disease severity and anticipated outcomes. These assessments were repeated on day 7 and at discharge. Time to peristaltic recovery, duration of fever, abdominal pain duration, and intra-abdominal pressure were monitored. Medical records were reviewed to determine mechanical ventilation, CRRT, vasoactive drug use, total hospital stay, ICU stay duration, and overall survival (OS) time from admission to death or last follow-up.
Statistical analysis was performed using SPSS 27.0 or Stata 15.0 software. Continuous data were described using mean and standard deviation (SD) for normally distributed data, while median and quartiles were used for non-normally distributed data. Independent samples t-tests were used for normally distributed and homogeneously variances data; otherwise, nonparametric tests (rank sum test) were applied. Categorical data were presented as absolute numbers or percentages and analyzed using chi-square tests. Logistic regression was used for multivariate analysis. Spearman correlation analysis assessed the relationship between FOS dosage and variables such as length of stay, ICU stay duration, SOFA score, WBC count, C-reactive protein, and PCT levels. Univariate logistic regression assessed the relationship between FOS dosage and binary outcomes (death and intra-abdominal infection). OS was analyzed using Kaplan–Meier method, with differences between survival curves assessed by log-rank test. A two-tailed p-value < 0.05 was considered statistically significant.
Results
Baseline characteristics
According to the Atlanta 2012 classification, 2993 cases (79.62%) of the patients were diagnosed with mild acute pancreatitis (MAP), 391 cases (10.40%) with moderate acute pancreatitis (MSAP), and 375 cases (9.98%) with SAP. Based on the inclusion and exclusion criteria of the study, a total of 110 patients were ultimately included in the research cohort, consisting of 75 males and 35 females, with an age range of 18–85 (47.55 ± 14.64) years. There were 73 patients in the TPF-normal group and 37 patients in the TPF-FOS group (Fig. 2). The demographic characteristics and baseline values of the patients on day 1 are shown in Table 1. There were no significant differences in baseline values between the two groups, including gender, age, Body Mass Index (BMI), causes of SAP, laboratory results, intra-abdominal pressure, and severity (P > 0.05).
Clinical outcomes of patients with SAP
Overall, the in-hospital mortality was statistically significant decreased in the TPF-FOS group compared with the TPF-normal group (13.50% vs. 34.20%, respectively; P = 0.021; Table 2). The length of ICU stay in the TPF-FOS group and TPF-normal group were 9.00 (4.00, 16.00) and 18.00 (11.00, 32.00) days, respectively (P < 0.001). There was no statistically significant difference in hospital stay duration between the TPF-FOS and TPF-normal groups (33.00 vs. 43.00, respectively; P = 0.142). Besides, the incidence of intra-abdominal infection, duration of fever, AGI score, time to peristaltic recovery, duration of abdominal pain and PCT level in the TPF-FOS group were significantly lower than those in the TPF-normal group (P < 0.05). The difference in the SOFA score between the TPF-FOS and TPF-normal groups on days 7 after admission was significant (4.00 vs. 6.00, respectively; P = 0.001). Compared with the TPF-normal group, lower levels of inflammation before discharge were observed in the TPF-FOS group, such as leukocytes, percentage of neutrophil, and C-reactive protein, but there were no significant difference between the two groups (P > 0.05). The ratios of mechanical ventilation, CRRT, and use of vasoactive drugs were lower in the TPF-FOS group than those in the TPF-normal group, and the differences were statistically significant (P < 0.05).
The potential effect of FOS feeding in patients with SAP
Kaplan Meier analysis was performed to explore the relationship between FOS supplemented EEN and in-hospital mortality with time dependence. Results indicated that there was no statistical difference in survival time between the TPF-FOS and TPF-normal groups (P = 0.240). However, this study found that TPF-FOS group indicated a trend to have a better prognosis while prolonged the hospital stays (Fig. 3). Next, we tried to explore the potential effect of FOS feeding in patients with SAP. Spearman correlation analysis and univariate logistic regression were performed to analyze the correlation between the outcomes in the TPF-FOS group and the FOS dosage. The results showed a significant negative correlation between FOS dosage and death (P = 0.019). Besides, a statistically significant association between the dose of FOS and intra-abdominal infection was observed (P = 0.043). There was no statistically significant association of the FOS dose with the length of stays, the length of ICU stays, WBC count, PCT level, C-reactive protein level or SOFA score before discharge (Table 3).

Survival curves by Kaplan–Meier analyses, the relationship between FOS supplemented EEN and in-hospital mortality.
During the study, 30 patients died (non-surviving group), and 80 patients survived (surviving group). Comparing the general data and blood biochemical indicators on day 1, the univariate analysis showed statistically significant differences in age (OR:1.065, 95% CI: 1.029–1.102, P = 0.001), blood urea nitrogen (OR: 1.101, 95% CI: 1.019–1.190, P = 0.015), serum sodium(OR: 1.105, 95% CI: 1.027–1.190, P = 0.008), triglycerides (OR: 0.927, 95% CI: 0.870–0.987, P = 0.019), FOS (OR: 0.847, 95% CI: 0.738–0.973, P = 0.019), NRS 2002 score (OR: 2.021, 95% CI: 1.331–3.071, P = 0.001), and APACHE II score (OR: 1.097, 95% CI: 1.009–1.191, P = 0.029) between the two groups (P < 0.05). Multivariable logistic regression showed that age was an independent risk factor for death (OR: 1.048, 95% CI: 1.008–1.090, P = 0.019), and FOS was an independent protective factor for death in patients with SAP (OR: 0.826, 95% CI: 0.687–0.993, P = 0.041) (Table 4).
Discussion
Despite a decline in the death rate over the past decade, SAP remains a high risk of mortality because of the advancement of pancreatic and extrapancreatic necrosis, the subsequent infection, and MODS. Mutinga et al. found that approximately half of the deaths from SAP were attributed to MODS in the early stages and were due to complications related to pancreatic necrosis in the late stages29.
In this study, the overall mortality rate was 27% (30 out of 110 patients). Importantly, the mortality rate was significantly lower in the TPF-FOS group compared to the TPF-normal group (13.50% vs. 34.20%). Both univariate and multivariate analyses identified FOS as a protective factor against death in patients with SAP. Furthermore, higher FOS dosage in EEN was associated with reduced mortality risk. These findings suggested that incorporating FOS into EEN could significantly decrease mortality and improve outcomes for patients with SAP.
The present study found that the incidence of intra-abdominal infection, length of fever, and WBC (on day 7), which reflected the levels of systemic inflammation, were significantly reduced in the TPF-FOS group compared to the TPF-normal group. The study suggested that it may be related to the physiological function of FOS. As a kind of SDF, FOS is fermented by intestinal bacteria to produce gas and short-chain fatty acids (SCFAs)30. On the one hand, SCFAs can stimulate the secretion of mucin by goblet cells. The mucin can improve the intestinal mucosal barrier function by strengthening the tight junctions of the intestinal epithelium31. Recent animal studies have found that in the absence of SDF, intestinal bacteria may erode the intestinal mucus barrier, and the integrity of the mucosal barrier is then damaged32. On the other hand, SCFAs can promote the proliferation of intestinal probiotics, such as Lactobacillus and Bifidobacterium. Intestinal probiotics can regulate intestinal flora, protect the intestinal mucosal barrier and reduce the level of the systemic inflammatory response33,34. Shinohara K. et al. found that the number of Bifidobacteria in the stools of healthy volunteers was increased, while Clostridium perfringens and Pseudomonas tended to decrease after the intake of SDF35. The decline in intestinal mucosal barrier function can lead to increased intestinal mucosal permeability, the translocation of intestinal flora, and the leakage of endotoxin into the blood circulation, which can exacerbate the systemic inflammatory response and MODS.
In this study, the AGI score, recovery of intestinal motility, and duration of abdominal pain were significantly lower in the TPF-FOS group following FOS supplementation. The Working Group on Abdomen (WGAP) of the European Society of Intensive Care Medicine (ESICM) defines AGI as gastrointestinal tract dysfunction in critically ill patients due to acute illness36. Reduced intestinal motility can lead to altered intestinal flora composition and potentially promote bacterial overgrowth, leading to bacterial translocation. These findings suggest that FOS may enhance gastrointestinal motility and improve gastrointestinal function. Similar conclusions have been observed where SDF improve bowel movements in conditions such as constipation, inflammatory bowel disease, irritable bowel syndrome, and diabetes11,12,13,14. The mechanisms involve gas production from FOS fermentation in the colon, directly stimulating intestinal peristalsis. Additionally, SCFAs produced by FOS fermentation promote 5-hydroxytryptamine secretion from colonic mucosal cells, stimulating intestinal nerves to enhance gastrointestinal motility31,37. Finally, SCFAs are the main energy source of intestinal epithelial cells16.
Results from this study indicated that on day 7, the SOFA score, PCT level, and length of ICU stay were significantly lower in the TPF-FOS group compared to the TPF-normal group. The SOFA score and PCT were noted as reliable indicators of disease severity and mortality risk in patients with SAP. Previous studies have suggested that reassessment of the SOFA score on the 7th day post-admission is valuable for predicting late mortality in SAP38,39. Hu et al. revealed that PCT may be a predictor of disease severity in COVID-1940. Laimoud et al. found that the SOFA score in patients with cardiogenic shock is a good predictor of hospital mortality, which was similar to the results of this study41. FOS can promote intestinal dysmotility, enhance intestinal mucosal barrier function, decrease intestinal mucosal permeability, alleviate endotoxin entry into blood, and decrease the systemic inflammatory response and MODS. Therefore, FOS can reduce the disease severity and improve the prognosis of SAP.
Mechanical ventilation can improve respiratory distress in patients with SAP. Gao et al. identified that CRRT can remove various inflammatory factors in SAP42. Vasoactive drugs can correct hypotension and maintain the blood perfusion to essential organs. In this study, we discovered that FOS reduced the rates of mechanical ventilation, CRRT, and use of vasoactive drugs in patients with SAP, which are crucial supportive treatments for critical patients. However, the mechanism is not known and may be related to the reduction in disease severity by FOS.
In addition, it has been shown that SDF can regulate insulin secretion and decrease triglycerides and glucose43,44. However, there was no significant difference in blood glucose and triglycerides between the TPF-FOS and TPF-normal groups in this study.
Gallstones and alcohol are the most prevalent causes of AP, but hypertriglyceridemia (HTG) is also a significant contributor, as seen in our study, where more than half of the patients had AP associated with HTG. In our study, the baseline triglyceride levels were lower in the EEN-FOS group, which may have influenced the outcomes observed in this group. Elevated triglyceride levels have been shown to contribute to pancreatic injury and worsen the course of AP45. In our study, the better prognosis in the EEN-FOS group may be attributed to both the reduction in triglyceride levels and the potential anti-inflammatory effects of FOS. However, apart from triglyceride levels, FOS may also play an important role in improving lipid metabolism, reducing pancreatic inflammation, and promoting intestinal recovery46. Although the results of this study may be particularly relevant for patients with hypertriglyceridemia-induced AP, we believe that the benefits of EEN-FOS combination therapy may extend beyond this subgroup. Future studies should further explore whether this treatment regimen is effective in other patient populations, including those without hypertriglyceridemia. Large-scale prospective trials will be needed to confirm the broader applicability of this treatment and elucidate the underlying mechanisms.
We acknowledge several limitations in our study. Firstly, the relatively small sample size, particularly in the TPF-FOS group, might introduce selection bias. Additionally, the lack of control over certain common variables poses a risk of collinearity bias. Due to the retrospective nature of our study, some data were missing, preventing us from determining the exact time interval from AP diagnosis to ICU admission for all patients. ICU transfer was based on clinical judgment, which varied with disease severity. The lack of a uniform ICU transfer criterion limits our ability to assess the impact of ICU timing on outcomes, and this warrants further investigation in future studies with more comprehensive data. Besides, the treatment decision between TPF and TPF-FOS was primarily based on the clinical judgment of physicians and the individual patient’s condition, the choice of treatment may have been influenced by various factors, introducing potential selection bias. Finally, our study indicated a possible role for FOS in influencing SAP outcomes. However, it is important to note that we did not directly assess the impact of FOS on the intestinal microbiota’s abundance or diversity in the two groups. Therefore, any potential influence of FOS on the proliferation of intestinal probiotics remains speculative and requires further empirical investigation. Moreover, the specific molecular mechanisms by which FOS may modulate the inflammatory response in SAP are not yet fully understood and warrant additional research. The molecular mechanism by which FOS reduces the inflammatory response in SAP remains to be clearly delineated.
While our findings suggest that FOS could potentially protect against mortality in patients with SAP, caution is warranted due to study limitations. The concept of FOS-supplemented EEN represents a promising intervention for improving prognosis in SAP. However, further research is necessary to establish its efficacy. Our study lays groundwork for future investigations and potential integration of FOS supplementation into SAP treatment protocols, underscoring the importance of larger, randomized controlled trials to validate these initial findings.
In conclusion, this study suggests that FOS may serve as an independent protective factor against mortality in SAP. Furthermore, supplementing EEN with FOS appears to contribute to reducing mortality and improving the prognosis of patients with SAP.
Data availability
All data generated or analysed during this study are included in this published article.
References
-
Banks, P. A. et al. Classification of acute pancreatitis–2012: revision of the Atlanta classification and definitions by international consensus. Gut 62, 102–111 (2013).
Google Scholar
-
van Santvoort, H. C. et al. A conservative and minimally invasive approach to necrotizing pancreatitis improves outcome. Gastroenterology 141, 1254–1263 (2011).
Google Scholar
-
Schepers, N. J., Besselink, M. G., van Santvoort, H. C., Bakker, O. J. & Bruno, M. J. Early management of acute pancreatitis. Best Pract. Res. Clin. Gastroenterol. 27, 727–743 (2013).
Google Scholar
-
Peng, L., Wu, L. G., Li, B., Zhao, J. & Wen, L. M. Early enteral nutrition improves intestinal immune barrier in a rat model of severe acute pancreatitis. J. Hepatobiliary Pancreat. Sci. 23, 681–687 (2016).
Google Scholar
-
Manohar, M., Verma, A. K., Venkateshaiah, S. U., Sanders, N. L. & Mishra, A. Pathogenic mechanisms of pancreatitis. World J. Gastrointest. Pharmacol. Ther. 8, 10–25 (2017).
Google Scholar
-
Lodewijkx, P. J. et al. Nutrition in acute pancreatitis: a critical review. Expert Rev. Gastroenterol. Hepatol. 10, 571–580 (2016).
Google Scholar
-
Ramanathan, M. & Aadam, A. A. Nutrition management in acute pancreatitis. Nutr. Clin. Pract. 34 (Suppl 1), S7–s12 (2019).
Google Scholar
-
Mederos, M. A. & Reber, H. A. Girgis M. D. Acute pancreatitis: a review. JAMA 325, 382–390 (2021).
Google Scholar
-
Jabłońska, B. & Mrowiec, S. Nutritional support in patients with severe acute pancreatitis-current standards. Nutrients 13 (2021).
-
Bejarano, N., Navarro, S., Rebasa, P., García-Esquirol, O. & Hermoso, J. Intra-abdominal pressure as a prognostic factor for tolerance of enteral nutrition in critical patients. JPEN J. Parenter. Enter. Nutr. 37, 352–360 (2013).
Google Scholar
-
Petrov, M. S. et al. Oral refeeding after onset of acute pancreatitis: a review of literature. Am. J. Gastroenterol. 102, 2079–2084 (2007). quiz 2085.
Google Scholar
-
Yu, T. et al. Effects of prebiotics and synbiotics on functional constipation. Am. J. Med. Sci. 353, 282–292 (2017).
Google Scholar
-
Wilson, B. & Whelan, K. Prebiotic inulin-type fructans and galacto-oligosaccharides: definition, specificity, function, and application in gastrointestinal disorders. J. Gastroenterol. Hepatol. 32 (Suppl 1), 64–68 (2017).
Google Scholar
-
Wang, X. et al. Physicochemical properties of the soluble dietary fiber from Laminaria Japonica and its role in the regulation of type 2 diabetes mice. Nutrients 14 (2022).
-
Tosh, S. M. & Bordenave, N. Emerging science on benefits of whole grain oat and barley and their soluble dietary fibers for heart health, glycemic response, and gut microbiota. Nutr. Rev. 78, 13–20 (2020).
Google Scholar
-
Wang, Y. et al. Comparative study on quality characteristics of Bischofia polycarpa seed oil by different solvents: lipid composition, phytochemicals, and antioxidant activity. Food Chem. X. 17, 100588 (2023).
Google Scholar
-
Chen, T. et al. Soluble dietary fiber reduces feeding intolerance in severe acute pancreatitis: a randomized study. JPEN J. Parenter. Enter. Nutr. 45, 125–135 (2021).
Google Scholar
-
Lian, P., Henricks, P. A. J., Wichers, H. J., Folkerts, G. & Braber, S. Differential effects of oligosaccharides, antioxidants, amino acids and PUFAs on heat/hypoxia-induced epithelial injury in a Caco-2/HT-29 co-culture model. Int. J. Mol. Sci. 24 (2023).
-
Liu, L. et al. Fructooligosaccharides improve growth performance and intestinal epithelium function in weaned pigs exposed to enterotoxigenic Escherichia coli. Food Funct. 11, 9599–9612 (2020).
Google Scholar
-
Shi, Y., Chen, F., Wang, Z., Cao, J. & Li, C. Effect and mechanism of functional compound fruit drink on gut microbiota in constipation mice. Food Chem. 401, 134210 (2023).
Google Scholar
-
Leppäniemi, A. et al. 2019 WSES guidelines for the management of severe acute pancreatitis. World J. Emerg. Surg. 14, 27 (2019).
Google Scholar
-
Beyer, G. et al. Clinical practice guideline—acute and chronic pancreatitis. Dtsch. Arztebl Int. 119, 495–501 (2022).
Google Scholar
-
Lakananurak, N. & Gramlich, L. Nutrition management in acute pancreatitis: clinical practice consideration. World J. Clin. Cases 8, 1561–1573 (2020).
Google Scholar
-
Liu, M. & Gao, C. A systematic review and meta-analysis of the effect of total parenteral nutrition and enteral nutrition on the prognosis of patients with acute pancreatitis. Ann. Palliat. Med. 10, 10779–10788 (2021).
Google Scholar
-
Malbrain, M. L., De laet, I., Viaene, D., Schoonheydt, K. & Dits, H. In vitro validation of a novel method for continuous intra-abdominal pressure monitoring. Intensive Care Med. 34, 740–745 (2008).
Google Scholar
-
Yuan, F., Damien, C. & Gaspard, N. Severity scores for status epilepticus in the ICU: systemic illness also matters. Crit. Care 27, 19 (2023).
Google Scholar
-
Hu, B. et al. Severity of acute gastrointestinal injury grade is a predictor of all-cause mortality in critically ill patients: a multicenter, prospective, observational study. Crit. Care 21, 188 (2017).
Google Scholar
-
Bargetzi, L. et al. Nutritional support during the hospital stay reduces mortality in patients with different types of cancers: secondary analysis of a prospective randomized trial. Ann. Oncol. 32, 1025–1033 (2021).
Google Scholar
-
Wolbrink, D. R. J. et al. Trends in early and late mortality in patients with severe acute pancreatitis admitted to ICUs: a nationwide cohort study. Crit. Care Med. 50, 1513–1521 (2022).
Google Scholar
-
Shimizu, K. et al. Gastrointestinal dysmotility is associated with altered gut flora and septic mortality in patients with severe systemic inflammatory response syndrome: a preliminary study. Neurogastroenterol. Motil. 23, 330–335 (2011). e157.
Google Scholar
-
Verspreet, J. et al. A critical look at prebiotics within the dietary fiber concept. Annu. Rev. Food Sci. Technol. 7, 167–190 (2016).
Google Scholar
-
Desai, M. S. et al. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell 167, 1339–1353e1321 (2016).
Google Scholar
-
Tinkov, A. A. et al. Gut as a target for cadmium toxicity. Environ. Pollut. 235, 429–434 (2018).
Google Scholar
-
Wang, R. et al. Modulation of intestinal barrier, inflammatory response, and gut microbiota by Pediococcus pentosaceus zy-B alleviates Vibrio parahaemolyticus infection in C57BL/6J mice. J. Agric. Food Chem. 70, 1865–1877 (2022).
Google Scholar
-
Shinohara, K., Ohashi, Y., Kawasumi, K., Terada, A. & Fujisawa, T. Effect of apple intake on fecal microbiota and metabolites in humans. Anaerobe 16, 510–515 (2010).
Google Scholar
-
Reintam Blaser, A. et al. Gastrointestinal function in intensive care patients: terminology, definitions and management. Recommendations of the ESICM Working Group on abdominal problems. Intensive Care Med. 38, 384–394 (2012).
Google Scholar
-
Bishehsari, F. et al. Dietary fiber treatment corrects the composition of gut microbiota, promotes SCFA production, and suppresses colon carcinogenesis. Genes (Basel) 9 (2018).
-
Tee, Y. S. et al. Serial evaluation of the SOFA score is reliable for predicting mortality in acute severe pancreatitis. Medicine (Baltim) 97, e9654 (2018).
Google Scholar
-
Gliem, N., Ammer-Herrmenau, C., Ellenrieder, V. & Neesse, A. Management of severe acute pancreatitis: an update. Digestion 102, 503–507 (2021).
Google Scholar
-
Hu, R., Han, C., Pei, S., Yin, M. & Chen, X. Procalcitonin levels in COVID-19 patients. Int. J. Antimicrob. Agents. 56, 106051 (2020).
Google Scholar
-
Laimoud, M. & Alanazi, M. The validity of SOFA score to predict mortality in adult patients with cardiogenic shock on venoarterial extracorporeal membrane oxygenation. Crit. Care Res. Pract 2020, 3129864 (2020).
-
Gao, N., Yan, C. & Zhang, G. Changes of serum procalcitonin (PCT), C-reactive protein (CRP), interleukin-17 (IL-17), interleukin-6 (IL-6), high mobility group protein-B1 (HMGB1) and D-dimer in patients with severe acute pancreatitis treated with continuous renal replacement therapy (CRRT) and its clinical significance. Med. Sci. Monit. 24, 5881–5886 (2018).
Google Scholar
-
Bader Ul Ain, H. et al. Effect of thermally treated barley dietary fiber against hypercholesterolemia. Food Sci. Nutr. 8, 5259–5266 (2020).
Google Scholar
-
Yu, K., Ke, M. Y., Li, W. H., Zhang, S. Q. & Fang, X. C. The impact of soluble dietary fibre on gastric emptying, postprandial blood glucose and insulin in patients with type 2 diabetes. Asia Pac. J. Clin. Nutr. 23, 210–218 (2014).
Google Scholar
-
Rajalingamgari, P. et al. Prospective observational study and mechanistic evidence showing lipolysis of circulating triglycerides worsens hypertriglyceridemic acute pancreatitis. J. Clin. Investig. 135, e184785 (2024).
Google Scholar
-
Paone, P. et al. Prebiotic oligofructose protects against high-fat diet-induced obesity by changing the gut microbiota, intestinal mucus production, glycosylation and secretion. Gut Microbes 14, 2152307 (2022).
Google Scholar
Funding
This study was supported by the Scientific Research Project of Hunan Provincial Health Commission (Grant No. 202210004958).
Author information
Authors and Affiliations
Contributions
Jing Qi designed this study. Fangchun Liu collected the data and wrote the manuscript. Zhiming Xiao critically revised the manuscript. Fangchun Liu and Zhiming Xiao contributed equally to this work. Hongyan Zeng, Jingbo Li and Feiyan Ai were involved in manuscript preparation. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This work has been carried out in accordance with the Declaration of Helsinki (2000) of the World Medical Association. This study was reviewed and approved by the Ethics Committee of the Third Xiangya Hospital of Central South University, with the approval number: Ethics No. 22138. Informed consent was not required for this study because of the retrospective study design and being established by our institutional review board policies.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
Reprints and permissions
About this article
Cite this article
Liu, F., Xiao, Z., Zeng, H. et al. Early enteral nutrition with fructooligosaccharides improves prognosis in severe acute pancreatitis.
Sci Rep 15, 5267 (2025). https://doi.org/10.1038/s41598-025-89739-x
-
Received: 15 December 2024
-
Accepted: 07 February 2025
-
Published: 12 February 2025
-
DOI: https://doi.org/10.1038/s41598-025-89739-x
Keywords
- Severe acute pancreatitis
- Early enteral nutrition
- Fructooligosaccharides
- Soluble dietary fiber
- Prognosis