Abstract
Uridine, a pyrimidine nucleoside, is crucial in the synthesis of metabolites. According to observational studies, a higher plasma uridine level is associated with a lower risk of atrial fibrillation (AF). However, the casual relationship between uridine and AF is still unknown. In this study, we used the Mendelian randomisation (MR) approach to explore causality. Three genetic variants associated with uridine were identified from the Metabolomics GWAS server (7824 participants); summary-level datasets associated with AF were acquired from a genome-wide association study (GWAS) meta-analysis with 1,030,836 European participants (60,620 AF cases). We duplicated the MR analyses using datasets from AF HRC studies and the FinnGen Consortium, and then conducted a meta-analysis which combined the main results. The risk of AF was significantly associated with the genetically determined plasma uridine level (odds ratio [OR] 0.27; 95% confidence interval [CI] 0.16, 0.47; p = 2.39 × 10–6). The association remained consistent in the meta-analysis of the various datasets (OR 0.27; 95% CI 0.17, 0.42; p = 1.34 × 10–8). In conclusion, the plasma uridine level is inversely associated with the risk of AF. Raising the plasma uridine level may have prophylactic potential against AF.
Introduction
Atrial fibrillation (AF), which is one of the most prevalent arrhythmias worldwide, increase the risk of stroke and embolism. The morbidity of AF is rising, due in part to the aging of populations worldwide1. According to data from the Framingham Heart Study, AF morbidity rates have increased threefold in the past 50 years2. For all that, a set of risk factors include high systolic blood pressure, body mass index, diabetes, and the like, have been proven to this day. The identification of protective factors for AF may facilitate the development of effective preventive and therapeutic measures.
Uridine, a pyrimidine nucleoside and regeneration-related metabolite, rejuvenates aged stem cells and regenerates tissues in vivo3. As a precursor of uridine triphosphate, uridine activates the synthesis of glycogen, participates in the synthesis of various other metabolites, and exerts positive effects on improving cardiac function4. Ion channel malfunction and aberrant Ca2+ handling are implicated in the pathogenesis of AF5. In experimental animals, the uridine derivative uridine-5ʹ-diphosphate reactivated the mitochondrial ATP-dependent potassium channel in the mitochondria of rat heart at 30 μM, thus ameliorating rhythm disorders6. Also, uridine promoted maintenance of mitochondrial homeostasis, thereby enhancing Ca2+ homeostasis in the myocardium4. Observational studies of the relationship between uridine and AF yielded discrepant results. No study has assessed the causal association between the plasma uridine level and AF, which might be clarified by large-scale randomised controlled trials (RCTs), however such trails are costly.
Mendelian randomisation (MR) is an epidemiological method that can reveal the causal association between exposure and outcome variables robustly7. MR is based on simulating an RCT with random assignment of variants during gametogenesis, and investigating causal associations taking account of minor residual confounders and reverse causality8,9. Specifically, single nucleotide polymorphisms (SNPs) are identified as unconfounded instrumental variables (IVs) in an MR study to proxy the phenotypes of interest7. The exposure and outcome datasets of a two-sample MR analysis should be obtained from two independent data sources to enhance reliability and validity9. Hence, based on large genome-wide association study (GWAS) datasets, we first explored the protective effect of uridine on AF using a two-sample MR approach.
Materials and methods
Study design
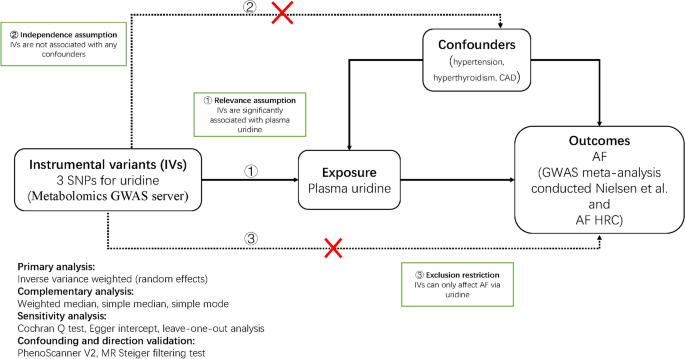
The causal relationship between uridine and the risk of AF was systematically investigated in a two-sample MR study. We selected SNPs as the IVs to proxy plasma uridine from a GWAS meta-analysis10, and confirmed the associations between IVs and AF in another large GWAS dataset11. There are three key assumptions12 that a convincing MR study must meet (Fig. 1). First, according to the relevance assumption, IVs should be significantly associated with plasma uridine; second, in light of the independence assumption, IVs should not be associated with confounders such as blood pressure, hyperthyroidism, or diabetes; third, in terms of the exclusion restriction, IVs should exert effects on the dependent variable (i.e. AF) only via (in this case) uridine. This study complied with The Strengthening the Reporting of Observational Studies in Epidemiology Using Mendelian Randomisation (STROBE-MR) standard (Table S1)13.

Mendelian randomisation framework diagram. SNPs single-nucleotide polymorphisms, AF atrial fibrillation, CAD coronary artery disease, AF HRC Atrial Fibrillation Haplotype Reference Consortium, GWAS genome-wide association study.
Ethic statement
All of the summary-level data used in this study are publicly available. Details regarding ethics approval and informed consent for each GWAS study included in this investigation can be found in the original publications.
Data source and SNP selection
Uridine-associated SNPs were identified from the Metabolomics GWAS server10, which included the Cooperative Health Research in the Region of Augsburg (KORA) study (1768 participants of European ancestry)14 and UK Adult Twin Registry (TwinsUK) cohort (6056 participants of European ancestry)15. Summary-level GWAS data for the association analysis between the uridine-associated genetic variants and AF were obtained from two large GWAS meta-analyses: (1) a primary meta-analysis of the Nord-Trøndelag Health Study (HUNT), deCODE, Michigan Genomics Initiative (MGI), DiscovEHR, UK Biobank, and AFGen Consortium, which encompassed 1,030,836 individuals of European descent (60,620 AF cases; and 970,216 controls)11 and (2) the Atrial Fibrillation Haplotype Reference Consortium (AF HRC), which comprised more than 50 studies (Table S2) including 588,190 individuals (65,446 AF cases; and 522,744 controls)16, mainly (91%) of European ancestry (Table 1). In the first GWAS dataset, AF was diagnosed according to the International Classification of Diseases (ICD)-9 or ICD-1011. In addition, we used the outcome datasets from the FinnGen Consortia17 to confirm the results of the analyses (Table 1). The sixth wave of GWAS data on AF was used, which comprised 28,670 cases (defined by ICD-8 code 42792, ICD-9 code 4273, and ICD-10 code I48) and 135,821 controls.
The Metabolomics GWAS Server provided 129 SNPs significantly associated with uridine at p < 1 × 10–510. To identify eligible genetic variants associated with uridine, a genome-wide significance threshold of p < 5 × 10–8 was set, excluding 118 SNPs that violated the relevance assumption. Next, to avoid bias from linkage disequilibrium (LD), we used the method of the 1000 Genomes European Project18 to perform clumping (r2, 0.001; clumping window, 10,000 kb)19. Then, we used PhenoScanner V220 to identify potential confounders (i.e. hypertension, hyperthyroidism, and coronary artery disease) significantly associated (p < 5 × 10−8) with the SNPs. To test the exclusion restriction assumption, SNPs with reverse causality were detected using the MR Steiger filtering method21 (Table S3). Finally, three SNPs associated with uridine were selected as the IVs (Table 2). Moreover, the F-statistic of each IV was calculated as a measure of weak instrumental variable bias, using the following formula: (mathrm{F}=frac{{mathrm{R}}^{2}times (mathrm{N}-k-1)}{1-{mathrm{R}}^{2}}), where R2 indicates the genetic variance in uridine explained by each SNP, N is the exposure dataset sample size, and k indicates the number of IVs22. IVs with an F-statistic less than 10 were considered too weak to represent the exposure and were thus removed23. The minor allele frequency of the IVs was not less than 0.01 which supported minor statistical bias due to low confidence.
Statistical analysis
We used the AF datasets from Nielsen et al. in the analysis of the primary outcome because of their large size and populations largely of European descent. The inverse variance weighted (IVW) method combined the Wald ratios of each valid SNP to calculate a pooled estimate, which is widely used in MR studies24. The main MR analysis method was the random-effects IVW method, which provides more reliable estimates when heterogeneity is present among SNPs25. To verify the robustness of the causal association between plasma uridine and AF, we replicated the IVW analysis using two AF GWAS from the AF HRC study and FinnGen Consortia aforementioned, and performed a meta-analysis to assess the overall situation. Also, a series of supplementary analyses were conducted, including weighted median26, simple median and simple mode27. Both the fixed-effects and random-effects models were considered for meta-analysis, depending on the heterogeneity among these MR results from different consortia. For meta-analysis, the p-value of Cochran’s Q statistic < 0.1 or I2 > 50% was deemed to be existing heterogeneity28,29.
For MR analysis, the I2 statistic and Cochran’s Q were calculated for the IVW model to assess heterogeneity among SNPs. If the p-value of Cochran’s Q was less than 0.05 or I2 was higher than 25%, we assumed heterogeneity among IVs30. In addition, pleiotropic bias was assumed to exist if the MR-Egger intercept’s p-value was less than 0.0531. To detect highly influential SNPs, we performed leave-one-out analysis32. The following formula was used to calculate the proportion of uridine phenotypic variance explained by each IV (R2): ({mathrm{R}}^{2}=frac{2 times mathrm{ EAF }times (1 -mathrm{ EAF}) times {mathrm{beta}}^{2}}{(2 times mathrm{ EAF }times (1 -mathrm{ EAF}) times {mathrm{beta}}^{2}) + 2 times mathrm{ EAF }times (1 -mathrm{ EAF}) times mathrm{ se }times mathrm{ N }times {mathrm{beta}}^{2})}). Here, the terms “EAF”, “beta”, “se”, and “N” stand for, respectively, “effect allele frequency”, “effect size”, “standard error”, and “sample size”30.
In light of the fact that this study involves multiple analyses, associations at a p-value (p < 0.017) after Bonferroni correction were deemed significant. We also calculated the power, analysis to determine the sample size required to assess the outcome measure, percentage of cases, sum of R2, and took 0.05 as the type I error rate in the online tool mRnd33. The MR analyses were conducted using the R software 4.2.234 with the TwoSampleMR package. And the meta package was used to perform meta-analysis.
Results
According to the results obtained by MR Steiger filtering (Table S3), three SNPs selected as the IVs explained more of the variance in plasma uridine levels than in AF, indicating that there was no reverse causality between the exposure and outcome variables. Together, these SNPs explained 20.3% of the phenotypic variability in plasma uridine (Table 2). The three SNPs had F-statistics higher than 30, which suggests there was a minor weak IVs bias in this study. In addition, the data sources for plasma uridine and AF have no sample overlap.
For the primary analysis, at an odds ratio (OR) of 0.27, the statistical power was 100% for detecting significant differences with a sample size of 1,030,836 (60,620 cases). According to the standard IVW analysis, higher genetically instrumented plasma uridine levels were significantly associated with a lower tendency to suffer from AF in both GWAS meta-analyses at a statistically significant level after Bonferroni correction (p < 0.017): (1) in the AF GWAS datasets conducted by Nielsen et al. (OR 0.27; 95% confidence interval [CI] 0.16, 0.47; p = 2.39 × 10–6) and (2) in the AF HRC (OR 0.26; 95% CI 0.11, 0.60; p = 1.59 × 10–3). Additionally, for the FinnGen Consortia, the MR estimate was positive, albeit nonsignificant and of relatively low magnitude (Figs. 2, 3). We included the three outcome datasets in a meta-analysis (OR 0.27; 95% CI 0.17, 0.42; p = 1.34 × 10–8), which demonstrated the reliability of results (Fig. 2). Several complementary statistical analyses with various assumptions were conducted to further support the primary results (Table 3). And the MR effect size for each SNP on AF was shown in the forest plot (Supplemental Fig. 1).

Association between genetically proxied plasma uridine level and the risk of AF. AF HRC Atrial Fibrillation Haplotype Reference Consortium, GWAS genome-wide association study, AF atrial fibrillation, OR odds ratio, CI confidence interval.

Scatter plot of five MR methods for assessing the association of the plasma uridine level with the risk of AF based on GWAS meta-analysis (Nielsen et al.) (A), AF HRC (B) and FinnGen (C). AF HRC Atrial Fibrillation Haplotype Reference Consortium, GWAS genome-wide association study, AF atrial fibrillation.
There was no evidence of heterogeneity or horizontal pleiotropy among the IVs, the p-values for Cochran’s Q and MR-Egger Intercept were much higher than 0.017 in both GWAS meta-analyses (Table 4). However, the I2 statistic calculated based on the FinnGen Consortium was 76%, indicating high heterogeneity among the SNPs. Therefore, we employed the random-effect IVW as the primary statistical approach to evaluate the causal relationship between plasma uridine level and the risk of AF. No single SNP significantly impacted the results in a leave-one-out analysis (Fig. 4).

MR leave-one-out analysis of the association of plasma uridine level with the risk of AF based on GWAS meta-analysis (Nielsen et al.) (A), AF HRC (B) and FinnGen (C). AF HRC Atrial Fibrillation Haplotype Reference Consortium, GWAS genome-wide association study, AF atrial fibrillation.
For meta-analysis, the p-value for Cochran’s Q was 0.922 and the I2 was 0%, indicating no heterogeneity among the MR results. The fixed-effects meta-analysis method is reliable in our studies.
Discussion
In this MR study, we first explored the causal association between plasma uridine levels and AF using publicly available GWAS datasets. We found evidence from a genetic perspective that an increased plasma uridine level reduces the risk of AF. Furthermore, we duplicated the results using a series of complementary MR methods. According to our analysis of the FinnGen Consortium dataset, there was high heterogeneity among IVs and an association of uridine with AF were directionally coherent (albeit lacking statistical significance). As the primary analysis approach, we applied the IVW method in the multiplicative random-effects model, which enables reliable estimation of heterogeneity among SNPs. Meta-analyses of the three datasets combined allowed us to obtain an integrated MR estimate, which facilitated causal inference. Our findings will aid the development of the novel preventive strategies for AF.
Uridine, a pyrimidine nucleoside, is more abundant in human plasma than other nucleosides35 and is critical in the synthesis of endogenous pyrimidine, glycogen, biomembranes and the like36. Uridine modulates heart rhythm disorders through its participation in myocardial glycogen resynthesis and ATP production for ion transport systems under pathological conditions37. Fibrosis has been well established and exerts a crucial effect in the development of AF38. Specifically, mitochondrial reactive oxygen species (ROS) are activated under various pathological and stress conditions, in turn activate p38 and ERK1/2 to accelerate the transcription of fibrotic genes39. Liu et al. found that uridine supplementation promoted tissue recovery in cardiac injury models, and decreased fibrosis and inflammatory cytokine levels3. Oxidative stress and inflammation play crucial roles in the onset and development of AF38. Additionally, uridine treatment can significantly decrease the levels of oxidative stress and inflammation in vitro40. The positive effects of uridine in reducing oxidative stress and inflammation might be involved with the activation of the mitoKATP channel40,41. And thus, it preserves the structure and function of mitochondria. Thereby ultimately ameliorating fibrosis in the myocardium.
Our findings coincide with a prospective cohort study based on the Atherosclerosis Risk in Communities study, which showed that a higher plasma uridine level was associated with a decreased risk of AF (hazard ratio 0.85; 95% CI 0.79, 0.92; p = 1.3 × 10–4)42. In addition, an observational cohort study based on the Framingham Heart Study yielded a similar, albeit nonsignificant, result regarding uridine as a protective factor for AF (hazard ratio 0.84; 95% CI 0.70, 1.00; p = 0.052)43. Observational studies can be influenced by potential confounders, which makes reverse causation difficult to determine and distorts true causal associations. To figure out whether uridine reduces the risk of AF, further prospective clinical trials based on large populations may shed light on this but expend a relative amount of time. This MR analysis based on datasets from GWASs of mountains of European population sought to demonstrate this issue. A recent MR investigation proposed similar conclusions with us. Their independent study extracted different IVs with a significant threshold at p < 1 × 10–5 and came to similar results44.
A strength of this study was its use of the MR analysis framework, which can reliably estimate causal associations. Also, our MR estimates strengthened the reliability of the casual inference between plasma uridine levels and AF with low confounding bias. Additionally, no horizontal pleiotropy was discovered for any of the outcomes examined using MR-Egger regression (a sensitivity analysis), MR Steiger filtering and PhenoScanner V2. There is thus a low probability of pleiotropic effects in this investigation. Our findings provide a foundation for further research on the prophylactic potential against AF of increasing plasma uridine levels.
However, this study had certain limitations worth recognizing. First, the quantity of uridine-associated SNPs was too few for performing the MR Pleiotropy Residual Sum and Outlier method, which were deemed a global test to access pleiotropy among IVs and provides an MR estimate after removing pleiotropic outliers45. It is still worth noting that a potential pleiotropy bias might have been present in this study. Second, the I2 statistic calculated based on the FinnGen Consortium was 76%, suggesting high heterogeneity among the three SNPs. This might be due to the amount of selected IVs was too few, larger GWAS datasets of uridine are needed to detect more uridine-associated SNPs. Third, given that this study was based on summary-level data, potential nonlinear associations between plasma uridine and AF were not examined. Fourth, the MR estimates based on the FinnGen Consortium database were significantly different from the others, which could be attributed to the fact that the AF cases in that database include some atrial flutter patients. Moreover, the AF HRC collaboration includes individuals of non-European ancestry (almost 9%), thus the demographic stratification may increase bias. Owing to this MR study was limited to participants who were mostly of European heritage, it is difficult to generalize to populations of other descents. Investigating the causal relationships among various populations would be of great interest. Future research is necessary to expand on our findings.
Conclusions
This MR study provided evidence from a genetic perspective suggesting that a higher plasma uridine level can reduce the propensity to suffer from AF. These results might open up new vistas for prevention strategies in AF.
Data availability
This study relied entirely on data from publicly available databases. The original data reported in the study are available in the article and Supplementary Materials. Inquiries should be addressed to the corresponding author.
Abbreviations
- AF:
-
Atrial fibrillation
- AF HRC:
-
Atrial Fibrillation Haplotype Reference Consortium
- CI:
-
Confidence interval
- EAF:
-
Effect allele frequency
- GWAS:
-
Genome-wide association study
- ICD:
-
International Classification of Diseases
- IVs:
-
Instrumental variables
- IVW:
-
Inverse-variance weighted
- KORA:
-
Cooperative Health Research in the Region of Augsburg
- LD:
-
Linkage disequilibrium
- MR:
-
Mendelian randomisation
- OR:
-
Odds ratio
- RCT:
-
Randomised controlled trials
- ROS:
-
Reactive oxygen species
- se:
-
Standard error
- SNPs:
-
Single nucleotide polymorphisms
- STROBE-MR:
-
Strengthening the reporting of observational studies in epidemiology using Mendelian randomisation
- TwinsUK:
-
UK Adult Twin Registry
References
-
Kornej, J., Börschel, C. S., Benjamin, E. J. & Schnabel, R. B. Epidemiology of atrial fibrillation in the 21st century: Novel methods and new insights. Circ. Res. 127, 4–20 (2020).
Google Scholar
-
Schnabel, R. B. et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: A cohort study. Lancet Lond. Engl. 386, 154–162 (2015).
Google Scholar
-
Liu, Z. et al. Cross-species metabolomic analysis identifies uridine as a potent regeneration promoting factor. Cell Discov. 8, 6 (2022).
Google Scholar
-
Belosludtseva, N. V. et al. Effect of chronic treatment with uridine on cardiac mitochondrial dysfunction in the C57BL/6 mouse model of high-fat diet-streptozotocin-induced diabetes. Int. J. Mol. Sci. 23, 10633 (2022).
Google Scholar
-
Andrade, J., Khairy, P., Dobrev, D. & Nattel, S. The clinical profile and pathophysiology of atrial fibrillation: Relationships among clinical features, epidemiology, and mechanisms. Circ. Res. 114, 1453–1468 (2014).
Google Scholar
-
Krylova, I. B. et al. The cardioprotective effect of uridine and uridine-5’-monophosphate: The role of the mitochondrial ATP-dependent potassium channel. Exp. Gerontol. 41, 697–703 (2006).
Google Scholar
-
Davey Smith, G. & Hemani, G. Mendelian randomization: Genetic anchors for causal inference in epidemiological studies. Hum. Mol. Genet. 23, R89-98 (2014).
Google Scholar
-
Holmes, M. V., Ala-Korpela, M. & Smith, G. D. Mendelian randomization in cardiometabolic disease: Challenges in evaluating causality. Nat. Rev. Cardiol. 14, 577–590 (2017).
Google Scholar
-
Zuccolo, L. & Holmes, M. V. Commentary: Mendelian randomization-inspired causal inference in the absence of genetic data. Int. J. Epidemiol. 46, 962–965 (2017).
Google Scholar
-
Shin, S.-Y. et al. An atlas of genetic influences on human blood metabolites. Nat. Genet. 46, 543–550 (2014).
Google Scholar
-
Nielsen, J. B. et al. Biobank-driven genomic discovery yields new insight into atrial fibrillation biology. Nat. Genet. 50, 1234–1239 (2018).
Google Scholar
-
Burgess, S. et al. Using published data in Mendelian randomization: A blueprint for efficient identification of causal risk factors. Eur. J. Epidemiol. 30, 543–552 (2015).
Google Scholar
-
Skrivankova, V. W. et al. Strengthening the reporting of observational studies in epidemiology using Mendelian randomization: The STROBE-MR statement. JAMA 326, 1614–1621 (2021).
Google Scholar
-
Suhre, K. et al. Human metabolic individuality in biomedical and pharmaceutical research. Nature 477, 54–60 (2011).
Google Scholar
-
Moayyeri, A., Hammond, C. J., Hart, D. J. & Spector, T. D. The UK Adult Twin Registry (TwinsUK Resource). Twin Res. Hum. Genet. 16, 144–149 (2013).
Google Scholar
-
Roselli, C. et al. Multi-ethnic genome-wide association study for atrial fibrillation. Nat. Genet. 50, 1225–1233 (2018).
Google Scholar
-
FinnGen_Consortium. FinnGen Data Freeze 6. https://www.finngen.fi/ (Accessed 31 October 2022) (2022).
-
1000 Genomes Project Consortium et al. A map of human genome variation from population-scale sequencing. Nature 467, 1061–1073 (2010).
Google Scholar
-
Hemani, G., Bowden, J. & Davey, S. G. Evaluating the potential role of pleiotropy in Mendelian randomization studies. Hum. Mol. Genet. 27, R195–R208 (2018).
Google Scholar
-
Kamat, M. A. et al. PhenoScanner V2: An expanded tool for searching human genotype-phenotype associations. Bioinform. Oxf. Engl. 35, 4851–4853 (2019).
Google Scholar
-
Hemani, G., Tilling, K. & Davey, S. G. Orienting the causal relationship between imprecisely measured traits using GWAS summary data. PLoS Genet. 13, e1007081 (2017).
Google Scholar
-
Papadimitriou, N. et al. Physical activity and risks of breast and colorectal cancer: A Mendelian randomisation analysis. Nat. Commun. 11, 597 (2020).
Google Scholar
-
Burgess, S., Thompson, S. G., CRP CHD Genetics Collaboration. Avoiding bias from weak instruments in Mendelian randomization studies. Int. J. Epidemiol. 40, 755–764 (2011).
Google Scholar
-
Burgess, S., Butterworth, A. & Thompson, S. G. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet. Epidemiol. 37, 658–665 (2013).
Google Scholar
-
Bowden, J. et al. A framework for the investigation of pleiotropy in two-sample summary data Mendelian randomization. Stat. Med. 36, 1783–1802 (2017).
Google Scholar
-
Bowden, J., Davey Smith, G., Haycock, P. C. & Burgess, S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet. Epidemiol. 40, 304–314 (2016).
Google Scholar
-
Hartwig, F. P., Davey Smith, G. & Bowden, J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int. J. Epidemiol. 46, 1985–1998 (2017).
Google Scholar
-
DerSimonian, R. & Laird, N. Meta-analysis in clinical trials. Control Clin. Trials 7, 177–188 (1986).
Google Scholar
-
Higgins, J. P. T. & Thompson, S. G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 21, 1539–1558 (2002).
Google Scholar
-
Greco, M. F. D., Minelli, C., Sheehan, N. A. & Thompson, J. R. Detecting pleiotropy in Mendelian randomisation studies with summary data and a continuous outcome. Stat. Med. 34, 2926–2940 (2015).
Google Scholar
-
Bowden, J., Davey Smith, G. & Burgess, S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 44, 512–525 (2015).
Google Scholar
-
Cheng, H., Garrick, D. J. & Fernando, R. L. Efficient strategies for leave-one-out cross validation for genomic best linear unbiased prediction. J. Anim. Sci. Biotechnol. 8, 38 (2017).
Google Scholar
-
Brion, M.-J.A., Shakhbazov, K. & Visscher, P. M. Calculating statistical power in Mendelian randomization studies. Int. J. Epidemiol. 42, 1497–1501 (2013).
Google Scholar
-
R_Core_Team. R: A Language and Environment for Statistical Computing. https://www.R-project.org/ (Accessed 31 October 2022) (R Foundation for Statistical Computing).
-
Yamamoto, T. et al. Biochemistry of uridine in plasma. Clin. Chim. Acta Int. J. Clin. Chem. 412, 1712–1724 (2011).
Google Scholar
-
Deng, Y. et al. An adipo-biliary-uridine axis that regulates energy homeostasis. Science 355, 5375 (2017).
Google Scholar
-
Bul’on, V. V. et al. Antiarrhythmic effect of uridine and uridine-5’-monophosphate in acute myocardial ischemia. Bull. Exp. Biol. Med. 157, 728–731 (2014).
Google Scholar
-
Sagris, M. et al. Atrial fibrillation: Pathogenesis, predisposing factors, and genetics. Int. J. Mol. Sci. 23, 6 (2021).
Google Scholar
-
Lu, H. et al. Danshensu inhibits β-adrenergic receptors-mediated cardiac fibrosis by ROS/p38 MAPK axis. Biol. Pharm. Bull. 37, 961–967 (2014).
Google Scholar
-
Jiang, N. & Zhao, Z. Intestinal aging is alleviated by uridine via regulating inflammation and oxidative stress in vivo and in vitro. Cell Cycle Georget Tex. 21, 1519–1531 (2022).
Google Scholar
-
Krylova, I. B. et al. Uridine treatment prevents myocardial injury in rat models of acute ischemia and ischemia/reperfusion by activating the mitochondrial ATP-dependent potassium channel. Sci. Rep. 11, 16999 (2021).
Google Scholar
-
Alonso, A. et al. Serum metabolomics and incidence of atrial fibrillation (from the atherosclerosis risk in communities study). Am. J. Cardiol. 123, 1955–1961 (2019).
Google Scholar
-
Ko, D. et al. Metabolomic profiling in relation to new-onset atrial fibrillation (from the Framingham Heart Study). Am. J. Cardiol. 118, 1493–1496 (2016).
Google Scholar
-
Cheng, T., Wang, H. & Hu, Y. The causal effects of genetically determined human blood metabolites on the risk of atrial fibrillation. Front. Cardiovasc. Med. 10, 1211458 (2023).
Google Scholar
-
Verbanck, M., Chen, C.-Y., Neale, B. & Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 50, 693–698 (2018).
Google Scholar
Acknowledgements
The authors appreciate the efforts of the staff and participants of the Metabolomics GWAS server, AF HRC, and FinnGen. They also thank Nielsen et al. for their work on the GWAS meta-analysis of atrial fibrillation. Additionally, they would like to express our gratitude to Heng Chen ([email protected]) for his MR training course. His excellent sharing of MR process makes it easier for us to accomplish this work.
Author information
Authors and Affiliations
Contributions
X.X.: Methodology, Investigation, Formal analysis, Software, Writing—original draft; X.Z.: Writing—review & editing; S.C.: Visualization, Formal analysis, Writing—review & editing; Q.L.: Validation, Resources; C.C.: Data Curation; M.O.: Conceptualization, Supervision. All of the authors contributed to and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Information.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
Reprints and Permissions
About this article
Cite this article
Xu, X., Zhang, X., Cheng, S. et al. Protective effect of uridine on atrial fibrillation: a Mendelian randomisation study.
Sci Rep 13, 19639 (2023). https://doi.org/10.1038/s41598-023-47025-8
-
Received: 15 January 2023
-
Accepted: 08 November 2023
-
Published: 10 November 2023
-
DOI: https://doi.org/10.1038/s41598-023-47025-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.