Abstract
Sex differences in cognitive function exist, but they are not stable and undergo dynamic change during the lifespan. However, our understanding of how sex-related neural information transmission evolves with age is still in its infancy. This study utilized the Wisconsin Card Sorting Test (WCST) and the label-free proteomics method with bioinformatic analysis to investigate the molecular mechanisms underlying age-related sex differences in cognitive performance in 199 healthy Thai subjects (aged 20–70 years), as well as explore the sex-dependent protein complexes for predicting cognitive aging. The results showed that males outperformed females in two of the five WCST sub-scores: %Corrects and %Errors. Sex differences in these scores were related to aging, becoming noticeable in those over 60. At the molecular level, differently expressed individual proteins and protein complexes between both sexes are associated with the potential N-methyl-D-aspartate type glutamate receptor (NMDAR)-mediated excitotoxicity, with the NMDAR complex being enriched exclusively in elderly female samples. These findings provided a preliminary indication that healthy Thai females might be more susceptible to such neurotoxicity, as evidenced by their cognitive performance. NMDAR protein complex enrichment in serum could be proposed as a potential indication for predicting cognitive aging in healthy Thai females.
Introduction
Cognitive function refers to the higher-order intellectual processes that gather and process information in the human brain. It contains multiple mental abilities, including language, memory, attention, problem-solving, and decision-making, to name a few1. At the molecular level, intact cognitive function is dependent on the precise exchange of information between neurons, which is initiated by the activation of excitatory neurotransmitter pathways2,3, primarily the glutamatergic pathway, because glutamate (Glu) is the major excitatory neurotransmitter in the central nervous system (CNS). However, its excessive activation would result in excitotoxicity that causes acute neuronal cell damage4,5, and Glu is considered a potent and fast-acting neurotoxin6. Clinical evidence has supported the role of excitotoxicity mediated by NMDAR, an ionotropic Glu receptor, in traumatic brain injury7,8 and cerebral ischemia9,10, both of which are acute CNS insults accompanied by cognitive deficits11,12. Furthermore, females are more frequently reported as patients with post-concussive symptoms, which are a combination of symptoms that occur after traumatic brain injury and include difficulty in concentrating and memory loss, in the existing literature than males13. Similarly, women experience more severe cerebral ischemic strokes and worse post-stroke cognitive impairments14,15.
In healthy populations, sex differences in cognitive function have been well studied16,17,18, but they are not stable and undergo dynamic change across the lifespan. According to Gur and Gur19, there was no correlation between age and any of the cognitive measures in women. Men, on the other hand, lost attention, verbal memory, spatial memory, and spatial abilities as they aged. Comparatively, in a longitudinal study of marmosets, cognitive impairment occurred earlier in females than in males, and it seems to be more prevalent for discrimination than in reversal learning20.
Our understanding of how sex-related neural information transmission evolves with age is still in its infancy. Chronic excitotoxicity has been related to Alzheimer’s disease21,22, which typically onsets in old age and is accompanied by cognitive deficits23, and has a different prevalence in men and women24. Recent research using mouse models revealed that male and female rodents had distinct excitatory glutamatergic synaptic inputs in both striatal brain regions and the hippocampus25,26, because estrogen regulates glutamatergic synaptic transmission by binding to different types of estrogen receptors27. Treatment with testosterone, on the other hand, enhanced the performance of aged male mice in the Morris water maze test and increased the level of NMDAR1 expression28. Furthermore, Glu-induced acute neurotoxicity in healthy individuals might be masked by the efficiency of normal cellular uptake mechanisms in removing Glu from the synaptic cleft6,29 and the potential neuroprotective effects of the sex steroids30,31. In other words, cognitive sex differences in healthy individuals are likely to be the phenotype of their susceptibility to the fast-acting and transient excitotoxicity induced by Glu because sex hormone regulation in the glutamatergic system changes with age, resulting in age-related cognitive sex differences.
Proteins perform most of the work in living cells, and changes in the expression of neurotransmission-related proteins resulted in age-dependent differences in male and female brain functioning32,33. To become functional, such neurotransmission-related proteins rapidly interacted with one another as protein complexes, which are functional units of proteome organization and are responsible for the majority of biological processes, such as biomedical pathways and signaling cascades in the neuron34,35. Synaptic transmission, for example, is dependent on transient and stable protein–protein interactions between the hundreds of components that form the pre- and post-synaptic compartments36,37. Instead of studying single proteins, researchers can gain a better understanding of the structure and function of the nervous system by studying protein complexes. Currently, clinical research is prone to using protein complexes to predict the mechanisms of neurodegenerative diseases such as Alzheimer’s disease (AD) and to develop novel treatments in response38,39,40.
Protein complexes are not permanent; their dynamic assembly is fundamental to inducing cellular responses to various internal and external stimuli, and the individual protein complexes involved in a signaling pathway assemble in different compartments at different times41. As a result, in order to gain a better understanding of how cells reorganize at a system level in the context of age-dependent cognitive sex differences, this study utilized both human behavior assessment (Wisconsin Card Sorting Test (WCST)) and molecular experiment (label-free proteomics analysis) at the protein global level to investigate the molecular mechanisms behind age-related cognitive sex differences in WCST performance in healthy men and women.
Results
Demographic data
The subjects were 89 males and 110 females, with a mean age of 45.6 ± 19.3 years (range 20–70 years). Age differed between men and women (BF = 10.7, 95% CI [2.74, 13.2]), and although a sex difference in education level was discovered (BF = 4.9), the evidence was moderate.
In each age group, differences in age and education level between men and women were not adequately supported. However, there were significant differences in education level among the three age groups (see Table 1).
Sex differences in cognitive performance
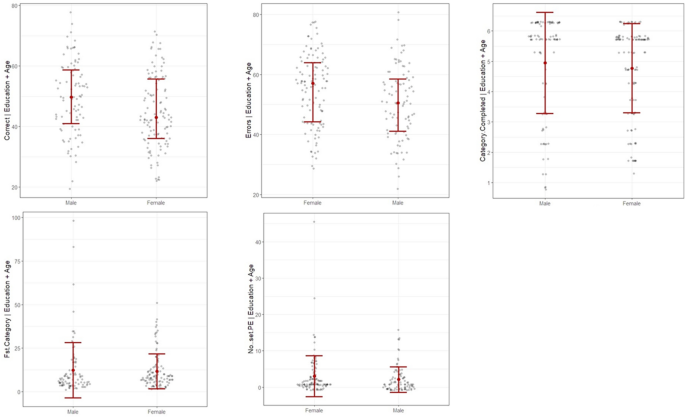
As shown in Fig. 1, males performed better in %Corrects and had fewer %Errors, with strong evidence to reject the null hypothesis. Weak evidence suggests the presence of sex differences in the other three scores: Category Completed, 1st Category, and PE. Table 2 shows the parameter estimation of sex disparities in those WCST sub-scores.

Sex differences in WCST sub-scores after controlling age and education level. Graphs were generated using flexplot package in R-programming ver. 4.1.2. Correct = %Corrects, Errors = %Errors, Fst Category = 1st Category, No..set PE = PE.
Changes in sex differences in WCST performance with age
Age-related sex differences were found in the same two WCST scores: %Corrects and %Errors. As shown in Fig. 2, males scored better in %Corrects (BF = 7.84, 95% CI [− 0.35, 12.6]) and had fewer total errors (BF = 7.58, 95% CI [− 12.7, 0.2]) in the young adult group; however, this sex difference reversed in the middle-aged adult group (%Corrects: BF = 2.09, 95% CI [− 1.27, 11.1]; %Errors: BF = 2.08, 95% CI [− 11.0, 1.54]). Nevertheless, there was insufficient evidence to support sex differences in these two scores in both young and middle-aged adult groups. While male dominance in %Corrects (BF > 100, 95% CI [4.32, 14.6], d = 0.89) and %Errors (BF > 100, 95% CI [− 14.6, − 4.29], d = 0.90) have been found in the elderly group, as in the young adult group, with decisive evidence to support it. In all three age groups, there was weak evidence to support the alternative hypothesis for the other three WCST scores: Category complete, 1st Category and PE (BF < 4).

Change of sex differences in WCST scores %Corrects and %Errors with age. Graphs were generated using flexplot package in R-programming ver. 4.1.2. Correct = %Corrects, Errors = %Errors.
Identification and relative quantification of differentially expressed proteins between males and females
The label-free proteomics analysis detected 19,640 proteins in each sample after applying protein FDR = 0.1. We discovered 52 proteins that were differentially expressed between male and female research subjects (FDR < 0.01) (see Table 3), with only 6 of those 52 differentially expressed proteins (DEPs) being upregulated in males (see Fig. 3).

Protein intensities comparison of those 6 DEPs upregulated in males. ***FDR ≤ 0.01.
Overrepresentation analysis of DEPs
To gain insight into the biological changes after activation of the glutamatergic system between both sexes. PANTHER overrepresentation analysis was employed to determine if such DEPs were enriched in certain groups based on the following three Gene annotation (GO) classes: molecular function (MF), biological process (BP), and cellular component (CC). The results showed that in the MF class, the DEPs involved in heparin binding (GO:0008201) were the most significantly enriched (Fold enrichment = 14, FDR = 0.025), and in the BP class, the DEPs involved in the regulation of complement activation (GO:0030449) were the most significantly overrepresented (Fold enrichment = 55.1, FDR = 0.016), while in the CC class, the DEPs involved in blood microparticle (GO:0072562) were the most significantly enriched (Fold enrichment = 22.4, FDR = 7.14E-6). See Fig. 4.

The top three processes in each GO class with DEPs significantly enriched.
Pathway enrichment analysis
The analysis of the KEGG pathways42,43,44 indicated that two significantly enriched pathways were found: complementing and coagulation cascades (has:04610) and Staphylococcus aureus infection (has:05150), see Table 4.
Protein–protein interaction analysis of DEPs
As illustrated in Fig. 5, 26 of the 52 DEPs interacted with one another, including protein complement 3 (C3), which is the central component of the complement system and is directly linked to the NMDA receptors (NMDARs). The data also suggested that the excitotoxicity induced by Glu could be mediated via NMDARs activity and estrogen-regulated NMDAR function. The majority of these 26 DEPs were shown to be upregulated in females (see Fig. 6).

Protein–protein interaction networks of 26 DEPs. The purple line indicates binding, the blue line indicates expression, the black line indicates chemical reactor, the solid gray indicates molecular transport, and the gray dotted line indicates regulation. The plus sign indicates positive regulation.

Expression levels of such 26 DEPs between males and females. The expression gradient is from green (minimum) to red (maximum).
Sex-dependent protein complexes analysis
To investigate the dynamic assembly of protein complexes stimulated by the WCST test, 19,640 previously identified proteins were submitted to the COMPLEAT database.
We found seven neurotransmission-related protein complexes selectively enriched in female samples: the elevation of cytosolic calcium ion concentration complex (P = 4.45E−04), activation of protein kinase activity complex (P = 6.99E−04), transmembrane receptor protein tyrosine kinase signaling pathway complex (4.38E−03), Multicomponent signaling complex (6.90E−03), positive regulation of kinase activity complex (P = 7.63E−03), JAK2-PAFR-TYK2 complex (7.82E−03), and receptor-mediated endocytosis complex (9.41E−03). In males, mucin type O-glycan biosynthesis complex (P = 6.52E−03), activation of phospholipase C activity complex (P = 3.98E−03), ULK1-ATG13-FIP200 complex (P = 1.31E−03), receptor-mediated endocytosis (P = 8.68E−03), NOTCH-core complex (P = 9.29E−03), and protein sumoylation complex (P = 9.06E−03) were selectively present, see Fig. 7.

Neurotransmission-related protein complexes are only enriched in males or females. Graph was generated on the website of COMPLEAT. Color gradient varies from red (max input score) to blue (min input score). Dashed lines indicate interactions.
The dynamic assembly of sex-dependent protein complexes with age
As shown in Fig. 8, we detected that the N-methyl-D-aspartate type glutamate receptor (NMDAR) complex (P = 0.005), regulation of protein kinase activity complex (P = 0.009), and G-protein coupled receptor signaling pathway complex (P = 0.008) were solely concentrated in elderly females. There were four neurotransmission-related complexes that were only enriched in elderly men: protein ubiquitination complex (P = 0.004), generation of neurons complex (P = 0.002), regulation of JUN kinases activity complex (P = 0.008), and negative regulation of apoptotic process complex (P = 0.007). Furthermore, the elevation of cytosolic calcium ion concentration complex (P < 0.005) was only found in females across all three age groups.

Neurotransmission-related protein complexes are enriched only in elderly males or females. Graph was generated on the website of COMPLEAT. Color gradient varies from red (max input score) to blue (min input score). Dashed lines indicate interactions.
The regulation of neuron differentiation complex (P = 0.003), NOTCH-Core complex (P = 0.0007), and activation of protein kinase activity complex (P = 0.0009) were found to be enriched only in females in the elderly samples; however, these complexes existed as common complexes in the middle-aged group and were not seen in young adult samples. While the axon guidance complex was present as a common complex in the young adult group, it was concentrated only in females in both middle-aged (P = 0.0007) and elderly (P = 0.002) groups. Furthermore, the nervous system development complex was found to be enriched only in males in both middle-aged (P = 0.009) and elderly (P = 0.008) groups, but not in young adults. The histone H3-K4 methylation complex was not present in the young adult group, was common in the middle-aged group, and was enriched only in males in the elderly group (P = 0.007).
Discussion
In the current study, to gain a better understanding of how cognitive sex differences evolve with age in healthy Thai subjects, frontal cortical cognitive performance was assessed using WCST. Males outperformed females in two of the five WCST sub-scores: %Corrects and %Errors, with a higher percentage of total corrects and a lower total error rate. We also found that the sex difference in these two WCST sub-scores changed with aging, and this difference becomes noticeable in those over 60. This is congruent with the findings of a study by Whitley, et al.45, which demonstrated that in UK residents, men and women subtraction scores and numerical problem-solving ability show different trends with aging beginning at 60.
The WCST is a test of cognitive flexibility, that is, the ability to adjust behavioral response mode in the face of changing conditions46,47, it is commonly used to test the function of the frontal cortex, particularly the prefrontal cortex48. Its sub-scores %Corrects and %Errors show specific domains of cognitive function, which correspond to the activity of certain brain regions. For example, %Corrects reflects conceptualization and attention, and a previous study showed that multiple areas of the prefrontal lobe may participate in information processing during an attentional shift in the WCST49. While %Errors reflecting non-specific cognitive impairment and lesions in the frontal and temporal lobes have been linked to a large number of WCST total errors50,51. This score has also been demonstrated to be adversely connected with the Full-Scale Intelligence Quotient (FSIQ)52,53, a measure of a person’s overall level of general cognitive and intellectual functioning54. Despite the standardization, validity, and reliability of the WCST as a stand-alone cognitive evaluation in healthy individuals of different ages and education levels have been established55, a battery of cognitive assessments is required to unveil the sex difference in different cognitive domains.
At the molecular level, the DEPs between sexes were most significantly enriched in complement cascade, which is a major component of the innate immune system56. One of the fundamental functions of complement activation in the healthy brain is to protect neurons from potentially harmful toxic stimuli57. In this study, data from protein–protein interaction analysis indicated that complement component 3 (C3), a central component of the complement system, was connected to NMDAR-mediated Glu-induced excitotoxicity. This conclusion is consistent with the previous finding that C3 was discovered to be expressed in the rat hippocampus following acute excitotoxic injury58. This result also demonstrated that estrogen, which may have a neuroprotective effect30,31,59, controls NMDAR function directly. Moreover, complement system components such as C3 and C4A, as well as regulators such as CFH, VTN, and SERPING 1, were detected in both males and females. Considering the blood drawn from the subjects after the WCST test, the complement activation is likely associated with the generation of potential excitotoxicity induced by Glu via NMDAR. The majority of the DEPs were upregulated in female subjects when compared to males, suggesting that females may be more susceptible to such excitotoxicity. as evidenced by their performance in score %Errors, which reflect nonspecific cognitive impairment and negatively connected with FSIQ.
To be functional, such DEPs are dynamically assembled as protein complexes, and this study uncovered a number of sex-biased protein complexes. For example, the elevation of the cytosolic calcium ion concentration complex was only found in the female samples. In classic excitotoxicity, impaired Glu transporter function leads to increased extracellular Glu, which elicits a massive influx of calcium into neurons via NMDARs60, and elevated calcium ions ultimately contribute to irreversible excitotoxic injury61. Protein kinase C (PKC), a family of protein kinase enzymes, has been shown to regulate NMDARs trafficking and gating62, and upregulating cellular PKC activity can exacerbate neurotoxicity mediated by NMDA receptor activation63. In addition, the platelet-activating factor receptor (PAFR) interacts with Tyk2 to promote Janus kinase 2 (Jak2) activation64. Jak is a type of protein tyrosine kinase65, and data from experimental mice and clinical observations have revealed multiple signaling events mediated by Jak in innate and adaptive immunity66.
Besides that, the activation of the phospholipase C (PLC) activity complex was identified only in males. PLC activation is associated with enhanced NMDAR function67, whereas PLC inhibition suppresses NMDAR-dependent long-term depression68. Another complex that is only found in men is the ULK1-ATG13-FIP200 complex, which mediates mTOR signaling69,70. A previous study has demonstrated that NMDAR activation regulates sociability through its effects on the mTOR signaling pathway71. Radiske et al.72 revealed that hippocampal NMDARs drive local protein synthesis via mTOR signaling and may control active memory maintenance, and that the enrichment of the ULK1-ATG13-FIP200 complex in males may offer a compensating effect on excitotoxicity-induced cell injury. Furthermore, protein sumoylation, as a post-translational modification, is essential in various biological processes, and an earlier study has shown that global sumoylation levels shape the immune responses73. This is consistent with the findings of the individual DEPs analysis and adds to its support. Additionally, previous research shows that one function of multi-component signaling is to mediate environmental stress74,75, and its selective enrichment in females suggests that the aforementioned sex-specific neurotransmission-related protein complexes are likely to be assembled transiently in response to the WCST stimulation. However, the DEPs were extracted from subjects’ blood rather than brain tissues; previous study indicated that plasma proteins permeate the healthy brain76, and that the majority of neurotransmission-related proteins are synthesized locally77,78. The data in the current study provides preliminary evidence that implies that differences in performances in WCST scores %Corrects and %Errors between male and female subjects might be due to their different susceptibilities to the potential NMDAR-mediated ecotoxicity.
Protein complexes are not permanent; their dynamic assembly is fundamental to inducing cellular responses to various internal and external stimuli, and the individual protein complexes involved in a signaling pathway assemble in different compartments at different times41. The N-methyl-D-aspartate type glutamate receptors (NMDARs) complex and its regulators tyrosine kinase (TK)79,80 and mitogen-activated protein kinase (MAPK)81, and G-protein coupled receptors82,83 were exclusively enriched in elderly females. While in elderly males, regulation of JUN kinase activity and protein ubiquitination complexes were only found in them. JUN kinase has been reported to mediate glutamate-induced excitotoxicity84, with JUN kinase controlling NMDAR-evoked presynaptic glutamate release as a possible mechanism85, and NMDAR activation leads to related protein ubiquitination86. This finding implied that subjects in the elderly group may have experienced higher levels of potential excitotoxicity mediated by NMDAR than the other two age groups. This is consistent with the hyperfunction theory of aging87, which states that aging is not functional decline but is caused by cellular hyperfunction—a function that was not switched off upon its completion—that results in age-related diseases. This theory linked aged-related functional loss to inappropriate activation of signaling pathways. Thus, later in life, although both elderly male and female subjects may experience higher-than-optimal levels of NMDAR functions, elderly females lose the neuroprotective effects of estrogen after menopause88,89, resulting in the loss of brain environment homeostasis and impaired cognitive function, as evidenced by their cognitive performance. This provided a possible explanation for why the sex differences in %Corrects and %Errors were only significant in the elderly group, and NMDAR protein complex enrichment in serum could be suggested as a potential indication for predicting cognitive aging in healthy Thai females.
There are several limitations to our study. First, in an attempt to obtain a sample that is approximately representative of the population, the sample covers a range of subjects with varied ages, education levels, socioeconomic statuses, and occupations; these also increase the variability of the sample. Second, except for age and education level, other confounders such as socioeconomic status and occupations were not controlled for in this study, despite the fact that both potentially contribute to the sex difference in cognitive performance. Third, only the subject’s education level was obtained; the number of years of study was not collected, and further research needs to better assess the contribution of years of education. Fourth, while some neurochemicals do cross the blood-brain barrier90, the majority of them might be locally synthesized in the brain91,92, and future studies directly comparing protein expression profiles in the brain tissues between males and females are needed to replicate our finding. Last, owing to the moderate sample size, generalizing the findings of this study should be done with caution.
Conclusion
The current study showed a preliminary connection between sex differences in cognitive performance in WCST in healthy Thai male and female subjects and their different susceptibilities to the potential NMDAR-mediated excitotoxicity that is modulated by sex steroids. Because of the efficiency with which molecular uptake mechanisms remove Glu from the synaptic cleft and neuroprotective effects of sex steroids, the acute and transient excitotoxicity in healthy populations might be masked; consequently, the WCST sub-score %Errors could be an indicator of potentially excitotoxic levels in them. Additionally, NMDAR protein complex enrichment in serum could be suggested as a potential indication for predicting cognitive aging in healthy Thai females. To our best knowledge, this is the first study to investigate the association between susceptibility to excitotoxicity and age-dependent cognitive sex differences in healthy populations, and our findings contribute to a better understanding of the neural mechanisms underlying the change of sex differences in cognitive functions with aging assessed by WCST.
Materials and methods
Study subjects
One hundred and ninety-nine healthy subjects aged 20–70 years were recruited, as described in a previous study from our lab93, and they were assigned into three age groups: (1) the young adult group, age range from 20 to 34 years (n = 70), (2) the middle-aged adult group, age range from 35 to 59 years (n = 59), and (3) the elderly group, age range from 60 years and above (n = 70). All subjects were of Thai ethnicity, to reduce the possibility of confounding by population stratification94. The research was approved by the Institutional Review Board (IRB) of Naresuan University, Thailand (COA No. 0262/2022). All methods were performed in accordance with relevant guidelines and regulations (Declaration of Helsinki). Participation was voluntary, and a written consent form was obtained from each participant involved.
Cognitive assessment
The cognitive performance of each research subject was assessed by the Wisconsin Card Sorting Test, specifically, five WCST sub-scores were used in this study: the percentage of total corrects (%Corrects), the percentage of total errors (%Errors), the number of categories completed (Category completed), the perseverative errors (PE), and trails to complete the first category (1st Category), as mentioned by a prior study93.
Blood sample collection
A 3 mL cubital vein blood sample was collected from each enrolled subject immediately after completing the WCST test, allowing us to explore the dynamic assembly of protein complexes in males and females from different age groups. Blood is an ideal biological sample for this study because it contains proteins from numerous cells and tissues and is convenient to assess95. The blood sample was centrifuged at 3000 rpm for 5 min. The serum was then transferred into a 1.5-ml microcentrifuge tube and stored at – 80 °C in a refrigerator for future use. All samples were coded to ensure anonymity.
Label-free quantitative proteomics analysis
As described by our previous study96, the label-free shotgun proteomics processes were performed by the Functional Proteomics Technology Laboratory, National Centre for Genetic Engineering and Biotechnology, Pathum Thani, Thailand, including protein preparation, peptide digestion, Liquid Chromatography with tandem mass spectrometry (LC–MS/MS) analysis, protein identification, and protein quantitation.
Briefly, five micrograms of protein samples were induced with 10 mM dithiothreitol, alkylated with 30 mM iodoacetamide, digested with sequencing grade porcine trypsin (1:20 ratio) for 16 h at 37 °C. The prepared tryptic peptide sample of each subject was injected individually into an Ultimate3000 Nano/Capillary LC System (Thermo Scientific, UK) coupled to a Hybrid quadrupole Q-Tof impact II™ (Bruker Daltonics) equipped with a Nano-captive spray ion source. Mass spectra (MS) and MS/MS spectra were obtained in the positive-ion mode at 2 Hz over the range (m/z) 150–2200. To minimize the effect of experimental variation, three independent MS/MS runs were performed for each sample.
MaxQuant 1.6.6.0 was used to quantify and identify the proteins in the individual samples using the Andromeda search engine to correlate MS/MS spectra to the Uniprot Homo sapiens database97. The proteins were identified by a 10% protein false discovery rate (FDR), carbamidomethylation of cysteine as fixed modification, and the oxidation of methionine and acetylation of the protein N-terminus as variable modifications. Only proteins with at least two peptides and at least one unique peptide were considered to be identified and used for further data analysis.
Bioinformatic analysis
Before any analysis, data cleansing and preprocessing were performed using Perseus 1.6.15.098. Differentially expressed proteins (DEPs) between both sexes were detected by the Linear Model for Microarray Data (LIMMA) approach within R-programming ver. 4.1.299, with FDR set at 1%.
PANTHER database analysis tools (ver. 16) were employed for the overrepresentation analysis of DEPs100. Pathway analysis was performed using the Database for Annotation, Visualization, and Integrated Discovery (DAVID) database101,102. The protein–protein interaction network was analyzed and visualized by Pathway Studio ver. 12.5103. The Complex Enrichment Analysis Tool (COMPLEAT), a web-based data mining and visualization tool for complex-based analysis of high-throughput data sets, as well as analysis and integration of heterogeneous proteomics and gene expression data sets104, was employed to investigate the dynamic assembly of protein complexes following the WCST in men and women of different ages. Expression levels of DEPs was visualized using Multi-Experiment Viewer software (MeV, ver.4.9.0)105. Benjamini corrected P-values less than 0.05 were considered significant.
Statistical analysis
Using R-programming ver. 4.1.2, a General Linear Model (GLM) approach paired with Bayesian statistics was applied to analyze sex differences in WCST sub-scores (covarying for age and educational level) and the difference of WCST scores between males and females from each age group (covarying for educational level)106. A Bayes factor ≥ 10 indicated a high likelihood of supporting the alternative hypothesis107.
The fold changes (FC) of DEPs were calculated and displayed as log2(FC).
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
-
Fisher, G. G., Chacon, M. & Chaffee, D. S. in Work Across the Lifespan (eds Boris B. Baltes, Cort W. Rudolph, & Hannes Zacher) 17–45 (Academic Press, 2019).
-
Cheng, Y. J., Lin, C. H. & Lane, H. Y. Involvement of cholinergic, adrenergic, and glutamatergic network modulation with cognitive dysfunction in Alzheimer’s disease. Int. J. Mol. Sci. https://doi.org/10.3390/ijms22052283 (2021).
Google Scholar
-
Xu, Y. et al. Neurotransmitter receptors and cognitive dysfunction in Alzheimer’s disease and Parkinson’s disease. Prog. Neurobiol. 97, 1–13. https://doi.org/10.1016/j.pneurobio.2012.02.002 (2012).
Google Scholar
-
Olney, J. W. Brain lesions, obesity, and other disturbances in mice treated with monosodium glutamate. Science 164, 719–721. https://doi.org/10.1126/science.164.3880.719 (1969).
Google Scholar
-
Olney, J. W. & Sharpe, L. G. Brain lesions in an infant rhesus monkey treated with monsodium glutamate. Science 166, 386–388. https://doi.org/10.1126/science.166.3903.386 (1969).
Google Scholar
-
Regan, R. F. & Choi, D. W. Glutamate neurotoxicity in spinal cord cell culture. Neuroscience 43, 585–591. https://doi.org/10.1016/0306-4522(91)90317-h (1991).
Google Scholar
-
Koura, S. S. et al. Relationship between excitatory amino acid release and outcome after severe human head injury. Acta Neurochir. Suppl. 71, 244–246. https://doi.org/10.1007/978-3-7091-6475-4_70 (1998).
Google Scholar
-
McIntosh, T. K., Vink, R., Soares, H., Hayes, R. & Simon, R. Effects of the N-methyl-D-aspartate receptor blocker MK-801 on neurologic function after experimental brain injury. J. Neurotrauma 6, 247–259. https://doi.org/10.1089/neu.1989.6.247 (1989).
Google Scholar
-
Jorgensen, M. B. & Diemer, N. H. Selective neuron loss after cerebral ischemia in the rat: Possible role of transmitter glutamate. Acta Neurol. Scand. 66, 536–546. https://doi.org/10.1111/j.1600-0404.1982.tb03140.x (1982).
Google Scholar
-
Thompson, R. J. Pannexin channels and ischaemia. J. Physiol. 593, 3463–3470. https://doi.org/10.1113/jphysiol.2014.282426 (2015).
Google Scholar
-
Hattori, K. et al. Cognitive deficits after focal cerebral ischemia in mice. Stroke 31, 1939–1944. https://doi.org/10.1161/01.str.31.8.1939 (2000).
Google Scholar
-
Hamm, R. J. et al. Cognitive deficits following traumatic brain injury produced by controlled cortical impact. J. Neurotrauma 9, 11–20. https://doi.org/10.1089/neu.1992.9.11 (1992).
Google Scholar
-
Farace, E. & Alves, W. M. Do women fare worse? A metaanalysis of gender differences in outcome after traumatic brain injury. Neurosurg. Focus 8, e6. https://doi.org/10.3171/foc.2000.8.1.152 (2000).
Google Scholar
-
Dong, L., Briceno, E., Morgenstern, L. B. & Lisabeth, L. D. Poststroke cognitive outcomes: Sex differences and contributing factors. J. Am. Heart Assoc. 9, e016683. https://doi.org/10.1161/JAHA.120.016683 (2020).
Google Scholar
-
Gall, S. L. et al. Sex differences in presentation, severity, and management of stroke in a population-based study. Neurology 74, 975–981. https://doi.org/10.1212/WNL.0b013e3181d5a48f (2010).
Google Scholar
-
Angrisani, M., Jain, U. & Lee, J. Sex differences in cognitive health among older adults in India. J. Am. Geriatr. Soc. 68(Suppl 3), S20–S28. https://doi.org/10.1111/jgs.16732 (2020).
Google Scholar
-
Ittig, S. et al. Sex differences in cognitive functioning in at-risk mental state for psychosis, first episode psychosis and healthy control subjects. Eur. Psychiatry 30, 242–250. https://doi.org/10.1016/j.eurpsy.2014.11.006 (2015).
Google Scholar
-
Levine, D. A. et al. Sex differences in cognitive decline among US adults. JAMA Netw. Open 4, e210169. https://doi.org/10.1001/jamanetworkopen.2021.0169 (2021).
Google Scholar
-
Gur, R. E. & Gur, R. C. Gender differences in aging: Cognition, emotions, and neuroimaging studies. Dialogues Clin. Neurosci. 4, 197–210. https://doi.org/10.31887/DCNS.2002.4.2/rgur (2002).
Google Scholar
-
Rothwell, E. S., Workman, K. P., Wang, D. & Lacreuse, A. Sex differences in cognitive aging: A 4-year longitudinal study in marmosets. Neurobiol. Aging 109, 88–99. https://doi.org/10.1016/j.neurobiolaging.2021.09.015 (2022).
Google Scholar
-
Ong, W. Y., Tanaka, K., Dawe, G. S., Ittner, L. M. & Farooqui, A. A. Slow excitotoxicity in Alzheimer’s disease. J. Alzheimers Dis. 35, 643–668. https://doi.org/10.3233/JAD-121990 (2013).
Google Scholar
-
Lewerenz, J. & Maher, P. Chronic glutamate toxicity in neurodegenerative diseases—What is the evidence?. Front. Neurosci. 9, 469. https://doi.org/10.3389/fnins.2015.00469 (2015).
Google Scholar
-
Bondi, M. W., Edmonds, E. C. & Salmon, D. P. Alzheimer’s disease: Past, present, and future. J. Int. Neuropsychol. Soc. 23, 818–831. https://doi.org/10.1017/S135561771700100X (2017).
Google Scholar
-
Li, R. & Singh, M. Sex differences in cognitive impairment and Alzheimer’s disease. Front. Neuroendocrinol. 35, 385–403. https://doi.org/10.1016/j.yfrne.2014.01.002 (2014).
Google Scholar
-
Cao, J., Willett, J. A., Dorris, D. M. & Meitzen, J. Sex differences in medium spiny neuron excitability and glutamatergic synaptic input: Heterogeneity across striatal regions and evidence for estradiol-dependent sexual differentiation. Front. Endocrinol. (Lausanne) 9, 173. https://doi.org/10.3389/fendo.2018.00173 (2018).
Google Scholar
-
Smith, M. D., Jones, L. S. & Wilson, M. A. Sex differences in hippocampal slice excitability: Role of testosterone. Neuroscience 109, 517–530. https://doi.org/10.1016/s0306-4522(01)00490-0 (2002).
Google Scholar
-
Oberlander, J. G. & Woolley, C. S. 17beta-Estradiol acutely potentiates glutamatergic synaptic transmission in the hippocampus through distinct mechanisms in males and females. J. Neurosci. 36, 2677–2690. https://doi.org/10.1523/JNEUROSCI.4437-15.2016 (2016).
Google Scholar
-
Jian-xin, J. et al. Effects of testosterone treatment on synaptic plasticity and behavior in senescence accelerated mice. J. Toxicol. Environ. Health A 78, 1311–1320. https://doi.org/10.1080/15287394.2015.1085839 (2015).
Google Scholar
-
Schousboe, A. Transport and metabolism of glutamate and GABA in neurons are glial cells. Int. Rev. Neurobiol. 22, 1–45. https://doi.org/10.1016/s0074-7742(08)60289-5 (1981).
Google Scholar
-
Burstein, S. R. et al. Estrogen receptor beta modulates permeability transition in brain mitochondria. Biochim. Biophys. Acta Bioenerg. 1859, 423–433. https://doi.org/10.1016/j.bbabio.2018.03.006 (2018).
Google Scholar
-
Mendelowitsch, A., Ritz, M. F., Ros, J., Langemann, H. & Gratzl, O. 17beta-Estradiol reduces cortical lesion size in the glutamate excitotoxicity model by enhancing extracellular lactate: A new neuroprotective pathway. Brain Res. 901, 230–236. https://doi.org/10.1016/s0006-8993(01)02359-9 (2001).
Google Scholar
-
Buck, S. A. et al. Vesicular glutamate transporter modulates sex differences in dopamine neuron vulnerability to age-related neurodegeneration. Aging Cell 20, e13365. https://doi.org/10.1111/acel.13365 (2021).
Google Scholar
-
Karoglu, E. T. et al. Aging alters the molecular dynamics of synapses in a sexually dimorphic pattern in zebrafish (Danio rerio). Neurobiol. Aging 54, 10–21. https://doi.org/10.1016/j.neurobiolaging.2017.02.007 (2017).
Google Scholar
-
Basu, A., Ash, P. E., Wolozin, B. & Emili, A. Protein interaction network biology in neuroscience. Proteomics 21, e1900311. https://doi.org/10.1002/pmic.201900311 (2021).
Google Scholar
-
Acuner Ozbabacan, S. E., Engin, H. B., Gursoy, A. & Keskin, O. Transient protein–protein interactions. Protein Eng. Des. Sel. 24, 635–648. https://doi.org/10.1093/protein/gzr025 (2011).
Google Scholar
-
Abul-Husn, N. S. et al. Systems approach to explore components and interactions in the presynapse. Proteomics 9, 3303–3315. https://doi.org/10.1002/pmic.200800767 (2009).
Google Scholar
-
Frank, R. A. W., Zhu, F., Komiyama, N. H. & Grant, S. G. N. Hierarchical organization and genetically separable subfamilies of PSD95 postsynaptic supercomplexes. J. Neurochem. 142, 504–511. https://doi.org/10.1111/jnc.14056 (2017).
Google Scholar
-
Soleimani Zakeri, N. S., Pashazadeh, S. & MotieGhader, H. Drug repurposing for Alzheimer’s disease based on protein-protein interaction network. Biomed. Res. Int. 2021, 1280237. https://doi.org/10.1155/2021/1280237 (2021).
Google Scholar
-
Zhang, Z. Q. et al. Increased prefrontal cortex connectivity associated with depression vulnerability and relapse. J. Affect. Disord. 304, 133–141. https://doi.org/10.1016/j.jad.2022.02.059 (2022).
Google Scholar
-
Shen, K. et al. Dual role of ribosome-binding domain of NAC as a potent suppressor of protein aggregation and aging-related proteinopathies. Mol. Cell 74, 729–741. https://doi.org/10.1016/j.molcel.2019.03.012 (2019).
Google Scholar
-
Hartwell, L. H., Hopfield, J. J., Leibler, S. & Murray, A. W. From molecular to modular cell biology. Nature 402, C47-52. https://doi.org/10.1038/35011540 (1999).
Google Scholar
-
Kanehisa, M. & Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28, 27–30. https://doi.org/10.1093/nar/28.1.27 (2000).
Google Scholar
-
Kanehisa, M. Toward understanding the origin and evolution of cellular organisms. Protein Sci. 28, 1947–1951. https://doi.org/10.1002/pro.3715 (2019).
Google Scholar
-
Kanehisa, M., Furumichi, M., Sato, Y., Kawashima, M. & Ishiguro-Watanabe, M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 51, D587-d592. https://doi.org/10.1093/nar/gkac963 (2023).
Google Scholar
-
Whitley, E. et al. Variations in cognitive abilities across the life course: Cross-sectional evidence from Understanding Society: The UK Household Longitudinal Study. Intelligence 59, 39–50. https://doi.org/10.1016/j.intell.2016.07.001 (2016).
Google Scholar
-
Nagahama, Y. et al. Age-related changes in cerebral blood flow activation during a Card Sorting Test. Exp. Brain Res. 114, 571–577. https://doi.org/10.1007/pl00005665 (1997).
Google Scholar
-
Nyhus, E. & Barcelo, F. The Wisconsin Card Sorting Test and the cognitive assessment of prefrontal executive functions: A critical update. Brain Cogn. 71, 437–451. https://doi.org/10.1016/j.bandc.2009.03.005 (2009).
Google Scholar
-
Konishi, S. et al. Transient activation of inferior prefrontal cortex during cognitive set shifting. Nat. Neurosci. 1, 80–84. https://doi.org/10.1038/283 (1998).
Google Scholar
-
Nagahama, Y. et al. Neural activity during attention shifts between object features. Neuroreport 9, 2633–2638. https://doi.org/10.1097/00001756-199808030-00038 (1998).
Google Scholar
-
Nelson, H. E. A modified card sorting test sensitive to frontal lobe defects. Cortex 12, 313–324. https://doi.org/10.1016/s0010-9452(76)80035-4 (1976).
Google Scholar
-
Horner, M. D., Flashman, L. A., Freides, D., Epstein, C. M. & Bakay, R. A. Temporal lobe epilepsy and performance on the Wisconsin Card Sorting Test. J. Clin. Exp. Neuropsychol. 18, 310–313. https://doi.org/10.1080/01688639608408285 (1996).
Google Scholar
-
Kopp, B., Maldonado, N., Scheffels, J. F., Hendel, M. & Lange, F. A meta-analysis of relationships between measures of Wisconsin Card Sorting and intelligence. Brain Sci. 9, 349 (2019).
Google Scholar
-
Lung, F. W., Chen, P. F. & Shu, B. C. Performance of Wisconsin Card Sorting Test in five-year-old children in Taiwan: Relationship to intelligence and cognitive development. PLoS One 13, e0202099. https://doi.org/10.1371/journal.pone.0202099 (2018).
Google Scholar
-
Lange, R. T. in Encyclopedia of Clinical Neuropsychology (eds Jeffrey S. Kreutzer, John DeLuca, & Bruce Caplan) 1103–1105 (Springer, 2011).
-
Miranda, A. R. et al. Age, education and gender effects on Wisconsin card sorting test: Standardization, reliability and validity in healthy Argentinian adults. Neuropsychol. Dev. Cogn. B Aging Neuropsychol. Cogn. 27, 807–825. https://doi.org/10.1080/13825585.2019.1693491 (2020).
Google Scholar
-
Ricklin, D., Hajishengallis, G., Yang, K. & Lambris, J. D. Complement: A key system for immune surveillance and homeostasis. Nat. Immunol. 11, 785–797. https://doi.org/10.1038/ni.1923 (2010).
Google Scholar
-
Ziabska, K., Ziemka-Nalecz, M., Pawelec, P., Sypecka, J. & Zalewska, T. Aberrant complement system activation in neurological disorders. Int. J. Mol. Sci. https://doi.org/10.3390/ijms22094675 (2021).
Google Scholar
-
Hernandez-Encinas, E. et al. Complement component 3 (C3) expression in the hippocampus after excitotoxic injury: Role of C/EBPbeta. J. Neuroinflammation 13, 276. https://doi.org/10.1186/s12974-016-0742-0 (2016).
Google Scholar
-
Zhao, L. & Brinton, R. D. Estrogen receptor alpha and beta differentially regulate intracellular Ca(2+) dynamics leading to ERK phosphorylation and estrogen neuroprotection in hippocampal neurons. Brain Res. 1172, 48–59. https://doi.org/10.1016/j.brainres.2007.06.092 (2007).
Google Scholar
-
Tymianski, M., Charlton, M. P., Carlen, P. L. & Tator, C. H. Source specificity of early calcium neurotoxicity in cultured embryonic spinal neurons. J. Neurosci. 13, 2085–2104. https://doi.org/10.1523/JNEUROSCI.13-05-02085.1993 (1993).
Google Scholar
-
Choi, D. W. Excitotoxicity: Still hammering the ischemic brain in 2020. Front. Neurosci. 14, 579953. https://doi.org/10.3389/fnins.2020.579953 (2020).
Google Scholar
-
Lan, J. Y. et al. Protein kinase C modulates NMDA receptor trafficking and gating. Nat. Neurosci. 4, 382–390. https://doi.org/10.1038/86028 (2001).
Google Scholar
-
Wagey, R., Hu, J., Pelech, S. L., Raymond, L. A. & Krieger, C. Modulation of NMDA-mediated excitotoxicity by protein kinase C. J. Neurochem. 78, 715–726. https://doi.org/10.1046/j.1471-4159.2001.00459.x (2001).
Google Scholar
-
Lukashova, V., Chen, Z., Duhe, R. J., Rola-Pleszczynski, M. & Stankova, J. Janus kinase 2 activation by the platelet-activating factor receptor (PAFR): Roles of Tyk2 and PAFR C terminus. J. Immunol. 171, 3794–3800. https://doi.org/10.4049/jimmunol.171.7.3794 (2003).
Google Scholar
-
Szilveszter, K. P., Nemeth, T. & Mocsai, A. Tyrosine kinases in autoimmune and inflammatory skin diseases. Front. Immunol. 10, 1862. https://doi.org/10.3389/fimmu.2019.01862 (2019).
Google Scholar
-
Ghoreschi, K., Laurence, A. & O’Shea, J. J. Janus kinases in immune cell signaling. Immunol. Rev. 228, 273–287. https://doi.org/10.1111/j.1600-065X.2008.00754.x (2009).
Google Scholar
-
Xiao, Z. et al. Requirement of phospholipase C and protein kinase C in cholecystokinin-mediated facilitation of NMDA channel function and anxiety-like behavior. Hippocampus 22, 1438–1450. https://doi.org/10.1002/hipo.20984 (2012).
Google Scholar
-
Horne, E. A. & Dell’Acqua, M. L. Phospholipase C is required for changes in postsynaptic structure and function associated with NMDA receptor-dependent long-term depression. J. Neurosci. 27, 3523–3534. https://doi.org/10.1523/JNEUROSCI.4340-06.2007 (2007).
Google Scholar
-
Ganley, I. G. et al. ULK1.ATG13.FIP200 complex mediates mTOR signaling and is essential for autophagy. J. Biol. Chem. 284, 12297–12305. https://doi.org/10.1074/jbc.M900573200 (2009).
Google Scholar
-
Hosokawa, N. et al. Nutrient-dependent mTORC1 association with the ULK1-Atg13-FIP200 complex required for autophagy. Mol. Biol. Cell 20, 1981–1991. https://doi.org/10.1091/mbc.e08-12-1248 (2009).
Google Scholar
-
Burket, J. A., Benson, A. D., Tang, A. H. & Deutsch, S. I. NMDA receptor activation regulates sociability by its effect on mTOR signaling activity. Prog Neuropsychopharmacol. Biol. Psychiatry 60, 60–65. https://doi.org/10.1016/j.pnpbp.2015.02.009 (2015).
Google Scholar
-
Radiske, A. et al. GluN2B and GluN2A-containing NMDAR are differentially involved in extinction memory destabilization and restabilization during reconsolidation. Sci. Rep. 11, 186. https://doi.org/10.1038/s41598-020-80674-7 (2021).
Google Scholar
-
Karhausen, J., Ulloa, L. & Yang, W. SUMOylation connects cell stress responses and inflammatory control: lessons from the gut as a model organ. Front. Immunol. 12, 646633. https://doi.org/10.3389/fimmu.2021.646633 (2021).
Google Scholar
-
Janeway, C. A. Jr. The T cell receptor as a multicomponent signalling machine: CD4/CD8 coreceptors and CD45 in T cell activation. Annu. Rev. Immunol. 10, 645–674. https://doi.org/10.1146/annurev.iy.10.040192.003241 (1992).
Google Scholar
-
Franchini, K. G., Torsoni, A. S., Soares, P. H. & Saad, M. J. Early activation of the multicomponent signaling complex associated with focal adhesion kinase induced by pressure overload in the rat heart. Circ. Res. 87, 558–565. https://doi.org/10.1161/01.res.87.7.558 (2000).
Google Scholar
-
Yang, A. C. et al. Physiological blood-brain transport is impaired with age by a shift in transcytosis. Nature 583, 425–430. https://doi.org/10.1038/s41586-020-2453-z (2020).
Google Scholar
-
Hafner, A. S., Donlin-Asp, P. G., Leitch, B., Herzog, E. & Schuman, E. M. Local protein synthesis is a ubiquitous feature of neuronal pre- and postsynaptic compartments. Science https://doi.org/10.1126/science.aau3644 (2019).
Google Scholar
-
Hillebrand, J., Barbee, S. A. & Ramaswami, M. P-body components, microRNA regulation, and synaptic plasticity. Sci. World J. 7, 178–190. https://doi.org/10.1100/tsw.2007.206 (2007).
Google Scholar
-
Salter, M. W. & Kalia, L. V. Src kinases: A hub for NMDA receptor regulation. Nat. Rev. Neurosci. 5, 317–328. https://doi.org/10.1038/nrn1368 (2004).
Google Scholar
-
Wang, Y. T. & Salter, M. W. Regulation of NMDA receptors by tyrosine kinases and phosphatases. Nature 369, 233–235. https://doi.org/10.1038/369233a0 (1994).
Google Scholar
-
Haddad, J. J. N-methyl-D-aspartate (NMDA) and the regulation of mitogen-activated protein kinase (MAPK) signaling pathways: A revolving neurochemical axis for therapeutic intervention?. Prog. Neurobiol. 77, 252–282. https://doi.org/10.1016/j.pneurobio.2005.10.008 (2005).
Google Scholar
-
Lutzu, S. & Castillo, P. E. Modulation of NMDA receptors by G-protein-coupled receptors: Role in synaptic transmission, plasticity and beyond. Neuroscience 456, 27–42. https://doi.org/10.1016/j.neuroscience.2020.02.019 (2021).
Google Scholar
-
MacDonald, J. F., Jackson, M. F. & Beazely, M. A. G protein-coupled receptors control NMDARs and metaplasticity in the hippocampus. Biochim. Biophys. Acta 1768, 941–951. https://doi.org/10.1016/j.bbamem.2006.12.006 (2007).
Google Scholar
-
Borsello, T. et al. A peptide inhibitor of c-Jun N-terminal kinase protects against excitotoxicity and cerebral ischemia. Nat. Med. 9, 1180–1186. https://doi.org/10.1038/nm911 (2003).
Google Scholar
-
Nistico, R. et al. Presynaptic c-Jun N-terminal kinase 2 regulates NMDA receptor-dependent glutamate release. Sci. Rep. 5, 9035. https://doi.org/10.1038/srep09035 (2015).
Google Scholar
-
Xu, J., Kurup, P., Nairn, A. C. & Lombroso, P. J. Synaptic NMDA receptor activation induces ubiquitination and degradation of STEP(61). Mol. Neurobiol. 55, 3096–3111. https://doi.org/10.1007/s12035-017-0555-x (2018).
Google Scholar
-
Blagosklonny, M. V. Aging and immortality: Quasi-programmed senescence and its pharmacologic inhibition. Cell Cycle 5, 2087–2102. https://doi.org/10.4161/cc.5.18.3288 (2006).
Google Scholar
-
Chompootweep, S., Tankeyoon, M., Yamarat, K., Poomsuwan, P. & Dusitsin, N. The menopausal age and climacteric complaints in Thai women in Bangkok. Maturitas 17, 63–71. https://doi.org/10.1016/0378-5122(93)90124-z (1993).
Google Scholar
-
Wariso, B. A. et al. Depression during the menopause transition: Impact on quality of life, social adjustment, and disability. Arch. Womens Ment. Health 20, 273–282. https://doi.org/10.1007/s00737-016-0701-x (2017).
Google Scholar
-
De Blasio, D. et al. Human brain trauma severity is associated with lectin complement pathway activation. J. Cereb. Blood Flow Metab. 39, 794–807. https://doi.org/10.1177/0271678X18758881 (2019).
Google Scholar
-
Veerhuis, R., Nielsen, H. M. & Tenner, A. J. Complement in the brain. Mol. Immunol. 48, 1592–1603. https://doi.org/10.1016/j.molimm.2011.04.003 (2011).
Google Scholar
-
Woodruff, T. M., Ager, R. R., Tenner, A. J., Noakes, P. G. & Taylor, S. M. The role of the complement system and the activation fragment C5a in the central nervous system. Neuromol. Med. 12, 179–192. https://doi.org/10.1007/s12017-009-8085-y (2010).
Google Scholar
-
Khanthiyong, B., Thanoi, S., Reynolds, G. P. & Nudmamud-Thanoi, S. Association study of the functional catechol-O-methyltranferase (COMT) Val(158)Met polymorphism on executive cognitive function in a Thai sample. Int. J. Med. Sci. 16, 1461–1465. https://doi.org/10.7150/ijms.35789 (2019).
Google Scholar
-
Freedman, M. L. et al. Assessing the impact of population stratification on genetic association studies. Nat. Genet. 36, 388–393. https://doi.org/10.1038/ng1333 (2004).
Google Scholar
-
Ray, S. et al. Proteomic technologies for the identification of disease biomarkers in serum: Advances and challenges ahead. Proteomics 11, 2139–2161. https://doi.org/10.1002/pmic.201000460 (2011).
Google Scholar
-
Chen, C. et al. Cholinergic-estrogen interaction is associated with the effect of education on attenuating cognitive sex differences in a Thai healthy population. PLoS One 18, e0278080. https://doi.org/10.1371/journal.pone.0278080 (2023).
Google Scholar
-
Tyanova, S., Temu, T. & Cox, J. The MaxQuant computational platform for mass spectrometry-based shotgun proteomics. Nat. Protoc. 11, 2301–2319. https://doi.org/10.1038/nprot.2016.136 (2016).
Google Scholar
-
Tyanova, S. et al. The perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods 13, 731–740. https://doi.org/10.1038/nmeth.3901 (2016).
Google Scholar
-
Smyth, G. K. in Bioinformatics and Computational Biology Solutions Using R and Bioconductor Statistics for Biology and Health (eds Robert Gentleman et al.) Ch. Chapter 23, 397–420 (Springer, 2005).
-
Mi, H. et al. PANTHER version 16: A revised family classification, tree-based classification tool, enhancer regions and extensive API. Nucleic Acids Res. 49, D394–D403. https://doi.org/10.1093/nar/gkaa1106 (2021).
Google Scholar
-
Sherman, B. T. et al. DAVID: a web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 50, W216–W221. https://doi.org/10.1093/nar/gkac194 (2022).
Google Scholar
-
da Huang, W., Sherman, B. T. & Lempicki, R. A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4, 44–57. https://doi.org/10.1038/nprot.2008.211 (2009).
Google Scholar
-
Nikitin, A., Egorov, S., Daraselia, N. & Mazo, I. Pathway studio—The analysis and navigation of molecular networks. Bioinformatics 19, 2155–2157. https://doi.org/10.1093/bioinformatics/btg290 (2003).
Google Scholar
-
Vinayagam, A. et al. Protein complex-based analysis framework for high-throughput data sets. Sci. Signal. 6, rs5. https://doi.org/10.1126/scisignal.2003629 (2013).
Google Scholar
-
Chu, V. T., Gottardo, R., Raftery, A. E., Bumgarner, R. E. & Yeung, K. Y. MeV+R: Using MeV as a graphical user interface for bioconductor applications in microarray analysis. Genome Biol. 9, R118. https://doi.org/10.1186/gb-2008-9-7-r118 (2008).
Google Scholar
-
Fife, D. The eight steps of data analysis: A graphical framework to promote sound statistical analysis. Perspect. Psychol. Sci. 15, 1054–1075. https://doi.org/10.1177/1745691620917333 (2020).
Google Scholar
-
Liang, F. & Xiong, M. Bayesian detection of causal rare variants under posterior consistency. PLoS One 8, e69633. https://doi.org/10.1371/journal.pone.0069633 (2013).
Google Scholar
Acknowledgements
The authors would like to thank all participants in this study. We would also like to thank the members of the Neuroscience and Reproductive Biological Research Group, Faculty of Medical Science, Naresuan University, Phitsanulok, Thailand and the staff of Proteomics Unit, The National Center for Genetic Engineering and Biotechnology, Pathum Thani, Thailand.
Author information
Authors and Affiliations
Contributions
C.C. contributed to conceptualization, data curation, methodology, formal analysis and the preparation of the original draft; B.K. contributed to data curation; B.T-S. contributed to data curation; S.C. contributed to methodology; S.R. contributed to methodology; S.T. contributed to conceptualization, manuscript editing, and approved the final draft of the manuscript; G.P.R contributed to conceptualization and manuscript editing; S.N-T. contributed to conceptualization, methodology, data analysis, supervision, manuscript editing and approved the final draft of the manuscript. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
Reprints and Permissions
About this article
Cite this article
Chen, C., Khanthiyong, B., Thaweetee-Sukjai, B. et al. Proteomic association with age-dependent sex differences in Wisconsin Card Sorting Test performance in healthy Thai subjects.
Sci Rep 13, 20238 (2023). https://doi.org/10.1038/s41598-023-46750-4
-
Received: 16 February 2023
-
Accepted: 04 November 2023
-
Published: 19 November 2023
-
DOI: https://doi.org/10.1038/s41598-023-46750-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.