Abstract
The purpose of this study was to evaluate how various parameters are related to microvasculature dropout (MvD) area measured using optical coherence tomography angiography (OCTA). We measured the area of MvD in 55 patients with primary open-angle glaucoma (POAG). Using OCTA, MvD area and peripapillary choroidal atrophy (PPA) area were assessed in a 4.5 mm × 4.5 mm region. The following were examined: circumpapillary nerve fiber layer (cpRNFL) thickness, optic disc area, optic disc cupping area, optic disc rim area, Humphrey Field Analyzer (HFA) 24/10–2 mean deviation (MD), and pattern standard deviation (PSD). The relationship between MvD area and each parameter was evaluated using Spearman’s rank correlation coefficient analysis. Mean MvD area and PPA area were 0.18 ± 0.17 mm2 and 1.13 ± 0.72 mm2, respectively. MvD area was significantly correlated with optic disc rim area (p = 0.0017), cpRNFL (p = 0.0027), HFA 24/10–2 MD, and PSD (p < 0.001). In eyes with POAG, MvD area indicates the severity of glaucoma, which might be associated with structural changes in the peripapillary vasculature around the optic disc.
Microvasculature dropout (MvD) refers to the loss of microvessels in the deep layers around the optic disc. It is often found in glaucoma1,2,3 and glaucoma with myopia4,5,6. In 2017, Lee et al. reported that MvD findings in the parapapillary choroid with OCT-angiography (OCTA) correspond to findings with ICG angiography7. This result suggests that MvD reflects the lack of blood flow in the deep layers. In addition, MvD has been observed at the site of optic neuropathy in primary open-angle glaucoma (POAG). Macular and optic nerve head superficial vessel densities are related to the progression of glaucomatous optic neuropathy8. Previous reports have shown that superficial blood flow is associated with thinning of the inner retinal layer and is a predictor of glaucoma progression8,9,10. In addition, deep blood flow loss can be observed with peripapillary atrophy in glaucoma, but these findings are highly correlated with thinning of the retinal nerve fiber layer and visual field disorder11,12. These findings are also related to paracentral visual field disorder13 and subsequent progression of glaucoma14. Furthermore, some studies have shown that peripapillary choroidal MvD is closely associated with intrapapillary circulatory disorders15,16.
We previously analyzed the relationships between structural indices such as surface blood vessel density and inner retinal layer thickness of the optic disc circumference17 or macula18 in eyes with glaucoma, functional indices such as central visual field sensitivity, and patients’ clinical characteristics. We concluded that a decrease in vascular density in the foveal avascular zone and peripapillary site is correlated with inner retinal layer thickness and central visual field impairment.
Many reports have described parapapillary MvD and glaucoma parameters, but the relationship between central 10° visual field and MvD has not been fully investigated. Therefore, to clarify the relationship between the structure and function of the central 10° visual field, which is important for visual acuity and vision quality in glaucoma, we investigated MvD area in patients with POAG. We evaluated the association between MvD area with each parameter to investigate its relationship to MvD using OCTA.
Methods
This study was approved by the ethics committee of Niigata University Medical and Dental Hospital (no. 2019-0055). The study was conducted in accordance with the Declaration of Helsinki. Informed consent was obtained from all patients, their legal guardians, or both.
Participants and examinations
This study included 55 eyes (29 right eyes and 26 left eyes) with POAG in 55 patients (29 men and 26 women) who underwent spectral-domain optical coherence tomography (SD-OCT) at Niigata University Medical and Dental Hospital between February 2018 and September 2021 to produce clear OCTA images of the papillary area. Mean age was 60.69 ± 12.18 years, mean spherical equivalent was − 3.91 ± 2.96 diopters (D), mean Humphrey field analyzer (HFA)10–2 mean deviation (MD) was − 10.03 ± 6.97 dB, and mean HFA24-2 MD was − 10.96 ± 6.37 dB (Table 1).
All patients underwent common ophthalmologic tests, including best-corrected visual acuity, refraction, corneal curvature, angle, intraocular pressure based on applanation tonometry, and visual field testing.
Diagnosis of POAG
Glaucoma was diagnosed by at least two glaucoma specialists. We referred to the fifth edition of the Japan Glaucoma Society Guidelines for Glaucoma19. The diagnostic criteria for POAG are as follows: (1) open-normal appearing anterior chamber angle and (2) glaucomatous visual field changes corresponding to glaucomatous optic disc changes, including retinal nerve fiber layer defects. Glaucomatous visual field changes met at least one of the following criteria: (1) three abnormal points, with a probability of being normal of p < 5% and one point with a pattern deviation of p < 1%, (2) a pattern SD or CPSD of p < 5%, or 3) values outside the normal limits in the glaucoma hemifield test. Glaucomatous optic neuropathy included at least one of the following: (1) vertical elongation of the optic cup, with an associated decrease in neuroretinal rim width (vertical cup-to-disc ratio ≥ 0.7, asymmetry ≥ 0.2); (2) notching of the neuroretinal rim; (3) disc hemorrhage; (4) parapapillary atrophy; (5) baring of the circumlinear vessel; and (6) exclusion of diseases that might cause glaucomatous changes in the optic disc. Inclusion criteria were: (1) POAG, (2) use of the Swedish Interactive Thresholding Algorithm (SITA) to acquire reliable test results (< 20% poor fixation and < 15% false-positive and false-negative rates), and (3) clear images obtained with Cirrus HD-OCT5000: Angiography 4.5 mm × 4.5 mm and Optic Cube 300 × 300, (4) signal strength index ≥ 7, and high-quality images were available for analysis.
Visual field was measured with a Humphrey Field Analyzer II (Carl Zeiss Meditec, Dublin, CA, USA) using the HFA central 10–2 and 24–2 SITA standard program test grid patterns. Only reliable test resultswere analyzed. Glaucomatous visual field defects were judged in accordance with Anderson and Patella’s standard20. When both eyes of a patient could have been included, the eye with worse visual field defects was included. Exclusion criteria were refractive values with astigmatism ≥ + 2D or ≤ − 2D, spherical equivalent ≤ − 8D, history of intraocular surgery, and history of eye disorders adversely affecting visual acuity or the visual field, including vascular occlusive diseases. The baseline characteristics of the study patients are shown in Table 1.
Measurement and determination of the presence of MvD
-
1.
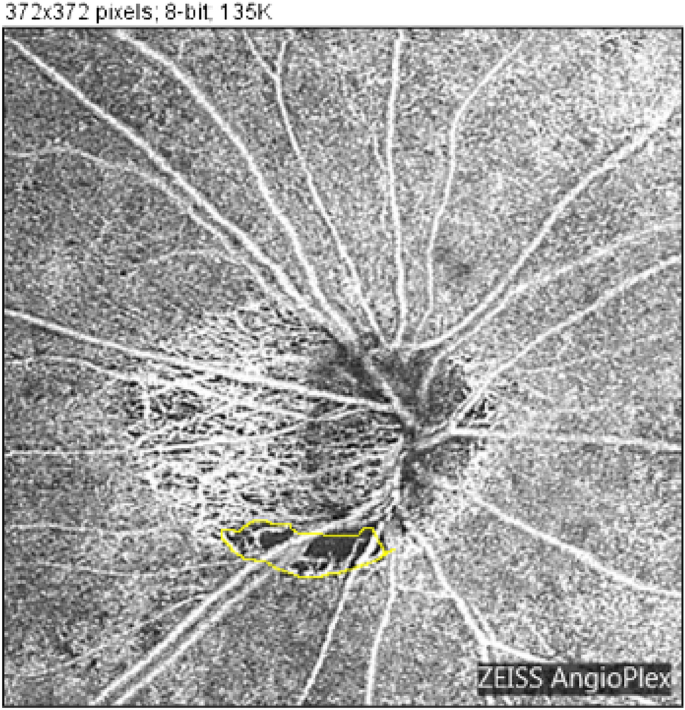
Determination of the presence of MvD: Peripapillary choroidal atrophy (PPA) was defined as deep choroidal atrophy around the optic disc. Inside the area of PPA, a defect of the microvascular shadow was defined as MvD (Fig. 1).
-
2.
Measurement of MvD: Two examiners independently measured MvD area in OCTA images defined in the previous section using ImageJ analysis software (ImageJ, United States National Institutes of Health)21,22. OCTA was performed using an HD-OCT (Cirrus HD-OCT5000 with AngioPlex OCT Angiography; Carl Zeiss Meditec). During optic nerve angiography, a 4.5 mm × 4.5 mm scan pattern was used to obtain images (Fig. 1). Images with signal strength index (an index of the reliability of images) ≤ 50 were excluded. MvD area was identified manually using ImageJ analysis software (Fig. 1, yellow line). MvD identification was performed at least once by each orthoptist (S.O. and D.M.), for a minimum of two measurements in total. They determined the final measurement values by averaging all measurements for analysis. From these images, the selected area was regarded as the MvD area and further analyzed. The first author reviewed all OCT images with an orthoptist (S.O.).

Manual measurement and computation of microvasculature dropout. Optical coherence tomography angiography (OCTA) was performed using HD-OCT (Cirrus HD-OCT5000 with AngioPlex OCT Angiography). Optic nerve angiography images were obtained using a 4.5 mm × 4.5 mm scan pattern. Images with signal strength index (an index of image reliability) ≤ 50 were excluded. Microvasculature dropout (MvD) area was identified manually using ImageJ analysis software (yellow line).
Statistical analysis
Relationships between MvD area and functional indices (measured sensitivity threshold (HFA10-2/24-2) MD and foveal threshold) were analyzed. Optic disc circumpapillary retinal nerve fiber layer (cpRNFL) thickness, PPA area, total optic disc area, optic disc rim area, and disc cupping area, all of which were determined with OCT, were considered structural indices. Age, spherical error (SE), and central corneal thickness (CCT), were considered clinical characteristics.
Spearman’s rank correlation coefficients were used to assess correlations between MvD area and functional and structural indices. We investigated these three types of parameters and their relationship to MvD area. For multivariable regression analysis, the forced entry method was performed with MvD area as the dependent variable; age, axial length, intraocular pressure (IOP), mean cpRNFL thickness, HFA 10-2/24-2 MD, and PPA area were included as independent variables. p < 0.05 was considered significant. Statistical analyses were performed with IBM SPSS Statistics for Windows, version 25.0 (IBM Corp., Armonk, NY).
Results
Intraobserver and interobserver reproducibility of MvD measurements
The intraclass correlation coefficients (ICCs) calculated using SPSS software were used to determine reproducibility as well as interobserver and intraobserver reliability of MvD measurements (Table 2). The intraobserver ICC for MvD was 0.983 (p < 0.001). The interobserver ICC (ICCmean) was 0.993 (p < 0.001) (Table 2).
Correlations between MvD and various parameters
MvD area, functional indices, structural indices, clinical characteristics, and their relationships are shown in Table 3. MvD area was 0.18 ± 0.17 mm2; 87.2% of all patients had findings of MvD. The overall mean optic disc rim area was 0.64 ± 0.19 µm. Mean cpRNFL thickness was 64.62 ± 9.68 µm. The overall foveal threshold (FT) was 35.36 ± 6.50 dB. Mean HFA10-2 MD was − 10.03 ± 7.22 dB and mean HFA24-2 MD was − 10.96 ± 6.43 dB. MvD area was significantly correlated with overall mean optic disc rim area (p = 0.01), mean cpRNFL thickness (p = 0.02), FT (p = 0.005), HFA10-2 MD (p < 0.0001), HFA24-2 MD (p < 0.0001), HFA10-2 PSD (p < 0.0001), and HFA24-2 PSD (p < 0.0001). Scatter plots show significant correlations between MvD area and structural indices (optic disc rim area and cpRNFL thickness) and functional indices (HFA10-2 MD, 24-2 MD, and FT) (Figs. 2 and 3).

Scatter plots showing correlations between microvasculature dropout and structural indices. The graphs show correlations between microvasculature dropout (MvD) area measured with optical coherence tomography angiography (OCTA) and rim area (A) and mean cpRNFL thickness (B), respectively. Spearman’s rank correlation coefficient analysis was used to assess correlations.

Scatter plots showing correlations between microvasculature dropout and functional indices. The graphs show correlations between microvasculature dropout (MvD) area measured using optical coherence tomography angiography (OCTA) and HFA10-2 MD (A), HFA24-2 MD (B), and foveal threshold (C), respectively. Spearman’s rank correlation coefficient analysis was used to assess correlations.
Clinical characteristics and their relationships with MvD area are shown in Table 3. Significant differences were only observed between MvD area and decimal visual acuity (VA) and between MvD area and the logarithm of the minimum angle of resolution (logMAR) VA.
Table 4 shows the results of multivariable regression analysis with MvD area as the dependent variable and age, mean axial length, IOP, mean cpRNFL thickness, HFA10-2 MD, and PPA area as independent variables.
Multivariable regression analysis using the simultaneous forced entry method revealed that MvD area is significantly associated with HFA10-2 MD (p = 0.001) and PPA area (p = 0.008).
Discussion
In this study, 87.2% of patients (48 of 55 eyes) had MvD. In a similar study using Avanti-XR by our department, 59% of patients (31 of 61 eyes) had MvD. Although the MvD detection rate was higher than in previous reports23, there might have been differences in patient characteristics. It is necessary to make a comparative study of patients with similar characteristics in the future. A recent study reported that MvD area and angular circumference change over time and are associated with concurrent cpRNFL loss in eyes with POAG24. Thus, it is possible that the patient population in this study included patients with severe disease.
In patients with MvD, functional indices (HFA24-2 MD, HFA10-2 MD, and average overall threshold sensitivity) and morphological indices (PPA area, thickness of each inner layer of the retina, and cpRNFL) were significantly lower than in patients without MvD. Furthermore, regarding clinical characteristics, there was a tendency towards older age and poorer eyesight; thus, it is possible that the finding of MvD is a characteristic of advanced glaucoma.
However, it is unclear whether MvD occurs because glaucoma has progressed or whether glaucoma progresses because of MvD. Recent prospective studies have shown that MvD is a predictor of progressive RNFL thinning in POAG, suggesting that MvD might have progressed earlier12.
MvD area was positively correlated with PPA area and negatively correlated with macular inner layer thickness, cpRNFL thickness, HFA24-2 MD, HFA10-2 MD, and visual field sensitivity threshold. Thus, MvD area might be related to structural changes around the optic disc as well as the structure of the inner layer of the retina, which might reflect the severity of glaucoma.
In a previous report, MvD was observed in PPA. PPA area and glaucoma severity were associated with the presence of MvD2. Therefore, circulation in the deep microvessels around the papilla is associated with glaucoma optic neuropathy.
This study had some limitations. Regarding MvD measurement, axial length and ocular refraction values were not corrected. Instead, we defined a population of extreme eye axes and reflex values as exclusion criteria from the beginning. We thought that no corrections that would significantly change the results were necessary. In addition, in multivariate analysis, eye axial length was corrected and analyzed. For these reasons, MvD and PPA areas were displayed in units of mm2 based on software measurements instead of pixel display, which might have affected accuracy.
Data availability
Data supporting the findings of the current study are available from the corresponding author upon reasonable request.
References
-
Akagi, T. et al. Microvascular density in glaucomatous eyes with hemifield visual field defects: An optical coherence tomography angiography study. Am. J. Ophthalmol. 168, 237–249. https://doi.org/10.1016/j.ajo.2016.06.009 (2016).
Google Scholar
-
Lee, E. J. et al. Parapapillary deep-layer microvasculature dropout in glaucoma: Topographic association with glaucomatous damage. Invest. Ophthalmol. Vis. Sci. 58(7), 3004–3010. https://doi.org/10.1167/iovs.17-21918 (2017).
Google Scholar
-
Suh, M. H. et al. Association between the deep-layer microvasculature dropout and the visual field damage in glaucoma. J. Glaucoma 27(6), 543–551. https://doi.org/10.1097/IJG.0000000000000961 (2018).
Google Scholar
-
Park, H. L., Jeon, S. J. & Park, C. K. Features of the choroidal microvasculature in peripapillary atrophy are associated with visual field damage in myopic patients. Am. J. Ophthalmol. 192, 206–216. https://doi.org/10.1016/j.ajo.2018.05.027 (2016).
Google Scholar
-
Na, H. M., Lee, E. J., Lee, S. H. & Kim, T. W. Evaluation of peripapillary choroidal microvasculature to detect glaucomatous damage in eyes with high myopia. J. Glaucoma 29(1), 39–45. https://doi.org/10.1097/IJG.0000000000001408 (2020).
Google Scholar
-
Kim, G. N., Lee, E. J. & Kim, T. W. Microstructure of nonjuxtapapillary microvasculature dropout in healthy myopic eyes. Invest. Ophthalmol. Vis. Sci. 61(2), 36. https://doi.org/10.1167/iovs.61.2.36 (2020).
Google Scholar
-
Lee, E. J. et al. Parapapillary choroidal microvasculature dropout in glaucoma: A comparison between optical coherence tomography angiography and indocyanine green angiography. Ophthalmology. 124(8), 1209–1217. https://doi.org/10.1016/j.ophtha.2017.03.039 (2017).
Google Scholar
-
Moghimi, S. et al. Macular and optic nerve head vessel density and progressive retinal nerve fiber layer loss in glaucoma. Ophthalmology 125(11), 1720–1728. https://doi.org/10.1016/j.ophtha.2018.05.006 (2018).
Google Scholar
-
Kim, J. A., Lee, E. J. & Kim, T. W. Evaluation of parapapillary choroidal microvasculature dropout and progressive retinal nerve fiber layer thinning in patients with glaucoma. JAMA Ophthalmol. 137(7), 810–816. https://doi.org/10.1001/jamaophthalmol.2019.1212 (2019).
Google Scholar
-
Kim, C. Y. et al. Progressive retinal nerve fibre layer thinning and choroidal microvasculature dropout at the location of disc haemorrhage in glaucoma. Br. J. Ophthalmol. 105(5), 674–680. https://doi.org/10.1136/bjophthalmol-2020-316169 (2021).
Google Scholar
-
Lin, S., Cheng, H. & Zhang, S. Parapapillary choroidal microvasculature dropout is associated with the decrease in retinal nerve fiber layer thickness: A prospective study. Invest. Ophthalmol. Vis. Sci. 60(2), 838–842. https://doi.org/10.1167/iovs.18-26115 (2019).
Google Scholar
-
Lee, E. J. et al. Influence of choroidal microvasculature dropout on the rate of glaucomatous progression: A prospective study. Ophthalmol. Glaucoma 3(1), 25–31. https://doi.org/10.1016/j.ogla.2019.10.001 (2020).
Google Scholar
-
Lee, E. J. et al. Central visual field damage and parapapillary choroidal microvasculature dropout in primary open-angle glaucoma. Ophthalmology 125(4), 588–596. https://doi.org/10.1016/j.ophtha.2017.10.036 (2018).
Google Scholar
-
Park, H. L., Kim, J. W. & Park, C. K. Choroidal microvasculature dropout is associated with progressive retinal nerve fiber layer thinning in glaucoma with disc hemorrhage. Ophthalmology 125(7), 1003–1013 (2018).
Google Scholar
-
Akagi, T. et al. Optic disc microvasculature dropout in primary open-angle glaucoma measured with optical coherence tomography angiography. PLoS One. 13(8), e0201729. https://doi.org/10.1371/journal.pone.0201729 (2018).
Google Scholar
-
Suh, M. H. et al. Optic disc microvasculature dropout in glaucoma detected by swept-source optical coherence tomography angiography. Am J Ophthalmol. 236, 261–270. https://doi.org/10.1016/j.ajo.2021.10.029 (2022).
Google Scholar
-
Igarashi, R. et al. Optical coherence tomography angiography of the peripapillary capillaries in primary open-angle and normal-tension glaucoma. PLoS One 12(9), e0184301. https://doi.org/10.1371/journal.pone.0184301 (2017).
Google Scholar
-
Igarashi, R. et al. Foveal avascular zone measurement via optical coherence tomography angiography and its relationship with the visual field in eyes with open-angle glaucoma. J Glaucoma. 29(6), 492–497. https://doi.org/10.1097/IJG.0000000000001492 (2020).
Google Scholar
-
JG Society. The Japan Glaucoma Society Guidelines for Glaucoma. Nippon Ganka Gakkai Zasshi 126(2), 85–177 (2003).
-
Anderson D & Patella V. Automated Static Perimetry. 2nd ed. Mosby (1999).
-
Rasband, W.S. ImageJ, U. S. National Institutes of Health, Bethesda, Maryland, USA, http://imagej.nih.gov/ij/, (1997–2012).
-
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 (2012).
Google Scholar
-
Sur, M. H. et al. Deep retinal layer microvasculature dropout detected by the optical coherence tomography angiography in glaucoma. Opthalmology 123(12), 2509–2518. https://doi.org/10.1016/j.ophtha.2016.09.002 (2016).
Google Scholar
-
Micheletti, E. et al. Rates of choroidal microvasculature dropout and retinal nerve fiber layer changes in glaucoma: Choroidal dropout and retinal nerve fiber layer changes. Am. J. Ophthalmol. 241, 130–138. https://doi.org/10.1016/j.ajo.2022.04.024 (2022).
Google Scholar
Author information
Authors and Affiliations
Contributions
Design and conduct of the study (R.I., S.O., T.F.), data collection (R.I., S.O., D.M., Y.S., R.I., T.F.), data management (R.I., S.O.), analysis (R.I., S.O.), interpretation of the data (R.I., T.A., T.F.), manuscript preparation (R.I.), review or approval of the manuscript (S.O., T.A., D.M., Y.S., R.I., T.F.).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
Reprints and Permissions
About this article
Cite this article
Igarashi, R., Ochiai, S., Akagi, T. et al. Parapapillary choroidal microvasculature dropout in eyes with primary open-angle glaucoma.
Sci Rep 13, 20601 (2023). https://doi.org/10.1038/s41598-023-48102-8
-
Received: 01 August 2023
-
Accepted: 22 November 2023
-
Published: 23 November 2023
-
DOI: https://doi.org/10.1038/s41598-023-48102-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.