Abstract
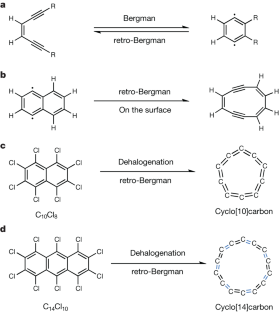
All-carbon materials based on sp2-hybridized atoms, such as fullerenes1, carbon nanotubes2 and graphene3, have been much explored due to their remarkable physicochemical properties and potential for applications. Another unusual all-carbon allotrope family are the cyclo[n]carbons (Cn) consisting of two-coordinated sp-hybridized atoms. They have been studied in the gas phase since the twentieth century4,5,6, but their high reactivity has meant that condensed-phase synthesis and real-space characterization have been challenging, leaving their exact molecular structure open to debate7,8,9,10,11. Only in 2019 was an isolated C18 generated on a surface and its polyynic structure revealed by bond-resolved atomic force microscopy12,13, followed by a recent report14 on C16. The C18 work trigged theoretical studies clarifying the structure of cyclo[n]carbons up to C100 (refs. 15,16,17,18,19,20), although the synthesis and characterization of smaller Cn allotropes remains difficult. Here we modify the earlier on-surface synthesis approach to produce cyclo[10]carbon (C10) and cyclo[14]carbon (C14) via tip-induced dehalogenation and retro-Bergman ring opening of fully chlorinated naphthalene (C10Cl8) and anthracene (C14Cl10) molecules, respectively. We use atomic force microscopy imaging and theoretical calculations to show that, in contrast to C18 and C16, C10 and C14 have a cumulenic and cumulene-like structure, respectively. Our results demonstrate an alternative strategy to generate cyclocarbons on the surface, providing an avenue for characterizing annular carbon allotropes for structure and stability.
This is a preview of subscription content, access via your institution
Access options
style{display:none!important}.LiveAreaSection-193358632 *{align-content:stretch;align-items:stretch;align-self:auto;animation-delay:0s;animation-direction:normal;animation-duration:0s;animation-fill-mode:none;animation-iteration-count:1;animation-name:none;animation-play-state:running;animation-timing-function:ease;azimuth:center;backface-visibility:visible;background-attachment:scroll;background-blend-mode:normal;background-clip:borderBox;background-color:transparent;background-image:none;background-origin:paddingBox;background-position:0 0;background-repeat:repeat;background-size:auto auto;block-size:auto;border-block-end-color:currentcolor;border-block-end-style:none;border-block-end-width:medium;border-block-start-color:currentcolor;border-block-start-style:none;border-block-start-width:medium;border-bottom-color:currentcolor;border-bottom-left-radius:0;border-bottom-right-radius:0;border-bottom-style:none;border-bottom-width:medium;border-collapse:separate;border-image-outset:0s;border-image-repeat:stretch;border-image-slice:100%;border-image-source:none;border-image-width:1;border-inline-end-color:currentcolor;border-inline-end-style:none;border-inline-end-width:medium;border-inline-start-color:currentcolor;border-inline-start-style:none;border-inline-start-width:medium;border-left-color:currentcolor;border-left-style:none;border-left-width:medium;border-right-color:currentcolor;border-right-style:none;border-right-width:medium;border-spacing:0;border-top-color:currentcolor;border-top-left-radius:0;border-top-right-radius:0;border-top-style:none;border-top-width:medium;bottom:auto;box-decoration-break:slice;box-shadow:none;box-sizing:border-box;break-after:auto;break-before:auto;break-inside:auto;caption-side:top;caret-color:auto;clear:none;clip:auto;clip-path:none;color:initial;column-count:auto;column-fill:balance;column-gap:normal;column-rule-color:currentcolor;column-rule-style:none;column-rule-width:medium;column-span:none;column-width:auto;content:normal;counter-increment:none;counter-reset:none;cursor:auto;display:inline;empty-cells:show;filter:none;flex-basis:auto;flex-direction:row;flex-grow:0;flex-shrink:1;flex-wrap:nowrap;float:none;font-family:initial;font-feature-settings:normal;font-kerning:auto;font-language-override:normal;font-size:medium;font-size-adjust:none;font-stretch:normal;font-style:normal;font-synthesis:weight style;font-variant:normal;font-variant-alternates:normal;font-variant-caps:normal;font-variant-east-asian:normal;font-variant-ligatures:normal;font-variant-numeric:normal;font-variant-position:normal;font-weight:400;grid-auto-columns:auto;grid-auto-flow:row;grid-auto-rows:auto;grid-column-end:auto;grid-column-gap:0;grid-column-start:auto;grid-row-end:auto;grid-row-gap:0;grid-row-start:auto;grid-template-areas:none;grid-template-columns:none;grid-template-rows:none;height:auto;hyphens:manual;image-orientation:0deg;image-rendering:auto;image-resolution:1dppx;ime-mode:auto;inline-size:auto;isolation:auto;justify-content:flexStart;left:auto;letter-spacing:normal;line-break:auto;line-height:normal;list-style-image:none;list-style-position:outside;list-style-type:disc;margin-block-end:0;margin-block-start:0;margin-bottom:0;margin-inline-end:0;margin-inline-start:0;margin-left:0;margin-right:0;margin-top:0;mask-clip:borderBox;mask-composite:add;mask-image:none;mask-mode:matchSource;mask-origin:borderBox;mask-position:0 0;mask-repeat:repeat;mask-size:auto;mask-type:luminance;max-height:none;max-width:none;min-block-size:0;min-height:0;min-inline-size:0;min-width:0;mix-blend-mode:normal;object-fit:fill;object-position:50% 50%;offset-block-end:auto;offset-block-start:auto;offset-inline-end:auto;offset-inline-start:auto;opacity:1;order:0;orphans:2;outline-color:initial;outline-offset:0;outline-style:none;outline-width:medium;overflow:visible;overflow-wrap:normal;overflow-x:visible;overflow-y:visible;padding-block-end:0;padding-block-start:0;padding-bottom:0;padding-inline-end:0;padding-inline-start:0;padding-left:0;padding-right:0;padding-top:0;page-break-after:auto;page-break-before:auto;page-break-inside:auto;perspective:none;perspective-origin:50% 50%;pointer-events:auto;position:static;quotes:initial;resize:none;right:auto;ruby-align:spaceAround;ruby-merge:separate;ruby-position:over;scroll-behavior:auto;scroll-snap-coordinate:none;scroll-snap-destination:0 0;scroll-snap-points-x:none;scroll-snap-points-y:none;scroll-snap-type:none;shape-image-threshold:0;shape-margin:0;shape-outside:none;tab-size:8;table-layout:auto;text-align:initial;text-align-last:auto;text-combine-upright:none;text-decoration-color:currentcolor;text-decoration-line:none;text-decoration-style:solid;text-emphasis-color:currentcolor;text-emphasis-position:over right;text-emphasis-style:none;text-indent:0;text-justify:auto;text-orientation:mixed;text-overflow:clip;text-rendering:auto;text-shadow:none;text-transform:none;text-underline-position:auto;top:auto;touch-action:auto;transform:none;transform-box:borderBox;transform-origin:50% 50%0;transform-style:flat;transition-delay:0s;transition-duration:0s;transition-property:all;transition-timing-function:ease;vertical-align:baseline;visibility:visible;white-space:normal;widows:2;width:auto;will-change:auto;word-break:normal;word-spacing:normal;word-wrap:normal;writing-mode:horizontalTb;z-index:auto;-webkit-appearance:none;-moz-appearance:none;-ms-appearance:none;appearance:none;margin:0}.LiveAreaSection-193358632{width:100%}.LiveAreaSection-193358632 .login-option-buybox{display:block;width:100%;font-size:17px;line-height:30px;color:#222;padding-top:30px;font-family:Harding,Palatino,serif}.LiveAreaSection-193358632 .additional-access-options{display:block;font-weight:700;font-size:17px;line-height:30px;color:#222;font-family:Harding,Palatino,serif}.LiveAreaSection-193358632 .additional-login>li:not(:first-child)::before{transform:translateY(-50%);content:””;height:1rem;position:absolute;top:50%;left:0;border-left:2px solid #999}.LiveAreaSection-193358632 .additional-login>li:not(:first-child){padding-left:10px}.LiveAreaSection-193358632 .additional-login>li{display:inline-block;position:relative;vertical-align:middle;padding-right:10px}.BuyBoxSection-683559780{display:flex;flex-wrap:wrap;flex:1;flex-direction:row-reverse;margin:-30px -15px 0}.BuyBoxSection-683559780 .box-inner{width:100%;height:100%}.BuyBoxSection-683559780 .readcube-buybox{background-color:#f3f3f3;flex-shrink:1;flex-grow:1;flex-basis:255px;background-clip:content-box;padding:0 15px;margin-top:30px}.BuyBoxSection-683559780 .subscribe-buybox{background-color:#f3f3f3;flex-shrink:1;flex-grow:4;flex-basis:300px;background-clip:content-box;padding:0 15px;margin-top:30px}.BuyBoxSection-683559780 .subscribe-buybox-nature-plus{background-color:#f3f3f3;flex-shrink:1;flex-grow:4;flex-basis:100%;background-clip:content-box;padding:0 15px;margin-top:30px}.BuyBoxSection-683559780 .title-readcube,.BuyBoxSection-683559780 .title-buybox{display:block;margin:0;margin-right:10%;margin-left:10%;font-size:24px;line-height:32px;color:#222;padding-top:30px;text-align:center;font-family:Harding,Palatino,serif}.BuyBoxSection-683559780 .title-asia-buybox{display:block;margin:0;margin-right:5%;margin-left:5%;font-size:24px;line-height:32px;color:#222;padding-top:30px;text-align:center;font-family:Harding,Palatino,serif}.BuyBoxSection-683559780 .asia-link{color:#069;cursor:pointer;text-decoration:none;font-size:1.05em;font-family:-apple-system,BlinkMacSystemFont,”Segoe UI”,Roboto,Oxygen-Sans,Ubuntu,Cantarell,”Helvetica Neue”,sans-serif;line-height:1.05em6}.BuyBoxSection-683559780 .access-readcube{display:block;margin:0;margin-right:10%;margin-left:10%;font-size:14px;color:#222;padding-top:10px;text-align:center;font-family:-apple-system,BlinkMacSystemFont,”Segoe UI”,Roboto,Oxygen-Sans,Ubuntu,Cantarell,”Helvetica Neue”,sans-serif;line-height:20px}.BuyBoxSection-683559780 .access-asia-buybox{display:block;margin:0;margin-right:5%;margin-left:5%;font-size:14px;color:#222;padding-top:10px;text-align:center;font-family:-apple-system,BlinkMacSystemFont,”Segoe UI”,Roboto,Oxygen-Sans,Ubuntu,Cantarell,”Helvetica Neue”,sans-serif;line-height:20px}.BuyBoxSection-683559780 .access-buybox{display:block;margin:0;margin-right:10%;margin-left:10%;font-size:14px;color:#222;opacity:.8px;padding-top:10px;text-align:center;font-family:-apple-system,BlinkMacSystemFont,”Segoe UI”,Roboto,Oxygen-Sans,Ubuntu,Cantarell,”Helvetica Neue”,sans-serif;line-height:20px}.BuyBoxSection-683559780 .price-buybox{display:block;font-size:30px;color:#222;font-family:-apple-system,BlinkMacSystemFont,”Segoe UI”,Roboto,Oxygen-Sans,Ubuntu,Cantarell,”Helvetica Neue”,sans-serif;padding-top:30px;text-align:center}.BuyBoxSection-683559780 .price-buybox-to{display:block;font-size:30px;color:#222;font-family:-apple-system,BlinkMacSystemFont,”Segoe UI”,Roboto,Oxygen-Sans,Ubuntu,Cantarell,”Helvetica Neue”,sans-serif;text-align:center}.BuyBoxSection-683559780 .price-info-text{font-size:16px;padding-right:10px;color:#222;font-family:-apple-system,BlinkMacSystemFont,”Segoe UI”,Roboto,Oxygen-Sans,Ubuntu,Cantarell,”Helvetica Neue”,sans-serif}.BuyBoxSection-683559780 .price-value{font-size:30px;font-family:-apple-system,BlinkMacSystemFont,”Segoe UI”,Roboto,Oxygen-Sans,Ubuntu,Cantarell,”Helvetica Neue”,sans-serif}.BuyBoxSection-683559780 .price-per-period{font-family:-apple-system,BlinkMacSystemFont,”Segoe UI”,Roboto,Oxygen-Sans,Ubuntu,Cantarell,”Helvetica Neue”,sans-serif}.BuyBoxSection-683559780 .price-from{font-size:14px;padding-right:10px;color:#222;font-family:-apple-system,BlinkMacSystemFont,”Segoe UI”,Roboto,Oxygen-Sans,Ubuntu,Cantarell,”Helvetica Neue”,sans-serif;line-height:20px}.BuyBoxSection-683559780 .issue-buybox{display:block;font-size:13px;text-align:center;color:#222;font-family:-apple-system,BlinkMacSystemFont,”Segoe UI”,Roboto,Oxygen-Sans,Ubuntu,Cantarell,”Helvetica Neue”,sans-serif;line-height:19px}.BuyBoxSection-683559780 .no-price-buybox{display:block;font-size:13px;line-height:18px;text-align:center;padding-right:10%;padding-left:10%;padding-bottom:20px;padding-top:30px;color:#222;font-family:-apple-system,BlinkMacSystemFont,”Segoe UI”,Roboto,Oxygen-Sans,Ubuntu,Cantarell,”Helvetica Neue”,sans-serif}.BuyBoxSection-683559780 .vat-buybox{display:block;margin-top:5px;margin-right:20%;margin-left:20%;font-size:11px;color:#222;padding-top:10px;padding-bottom:15px;text-align:center;font-family:-apple-system,BlinkMacSystemFont,”Segoe UI”,Roboto,Oxygen-Sans,Ubuntu,Cantarell,”Helvetica Neue”,sans-serif;line-height:17px}.BuyBoxSection-683559780 .tax-buybox{display:block;width:100%;color:#222;padding:20px 16px;text-align:center;font-family:-apple-system,BlinkMacSystemFont,”Segoe UI”,Roboto,Oxygen-Sans,Ubuntu,Cantarell,”Helvetica Neue”,sans-serif;line-height:NaNpx}.BuyBoxSection-683559780 .button-container{display:flex;padding-right:20px;padding-left:20px;justify-content:center}.BuyBoxSection-683559780 .button-container>*{flex:1px}.BuyBoxSection-683559780 .button-container>a:hover,.Button-505204839:hover,.Button-1078489254:hover,.Button-2496381730:hover{text-decoration:none}.BuyBoxSection-683559780 .readcube-button{background:#fff;margin-top:30px}.BuyBoxSection-683559780 .button-asia{background:#069;border:1px solid #069;border-radius:0;cursor:pointer;display:block;padding:9px;outline:0;text-align:center;text-decoration:none;min-width:80px;margin-top:75px}.BuyBoxSection-683559780 .button-label-asia,.ButtonLabel-3869432492,.ButtonLabel-3296148077,.ButtonLabel-1651148777{display:block;color:#fff;font-size:17px;line-height:20px;font-family:-apple-system,BlinkMacSystemFont,”Segoe UI”,Roboto,Oxygen-Sans,Ubuntu,Cantarell,”Helvetica Neue”,sans-serif;text-align:center;text-decoration:none;cursor:pointer}.Button-505204839,.Button-1078489254,.Button-2496381730{background:#069;border:1px solid #069;border-radius:0;cursor:pointer;display:block;padding:9px;outline:0;text-align:center;text-decoration:none;min-width:80px;max-width:320px;margin-top:10px}.Button-505204839 .readcube-label,.Button-1078489254 .readcube-label,.Button-2496381730 .readcube-label{color:#069}
/* style specs end */
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Rent or buy this article
Prices vary by article type
from$1.95
to$39.95
Prices may be subject to local taxes which are calculated during checkout




Data availability
All data supporting the findings of this study are available within the paper and its online Extended Data files.
References
-
Kroto, H. W., Heath, J. R., O’Brien, S. C., Curl, R. F. & Smalley, R. E. C60: buckminsterfullerene. Nature 318, 162–163 (1985).
Google Scholar
-
Iijima, S. & Ichihashi, T. Single-shell carbon nanotubes of 1-nm diameter. Nature 363, 603–605 (1993).
Google Scholar
-
Novoselov, K. S. et al. Electric field effect in atomically thin carbon films. Science 306, 666–669 (2004).
Google Scholar
-
Parent, D. C. & McElvany, S. W. Investigations of small carbon cluster-ion structures by reactions with hydrogen cyanide. J. Am. Chem. Soc. 111, 2393–2401 (1989).
Google Scholar
-
Van Orden, A. & Saykally, R. J. Small carbon clusters: spectroscopy, structure, and energetics. Chem. Rev. 98, 2313–2357 (1998).
Google Scholar
-
Grutter, M. et al. Electronic absorption spectra of linear C6, C8 and cyclic C10, C12 in neon matrices. J. Chem. Phys. 111, 7397–7401 (1999).
Google Scholar
-
Pitzer, K. S. & Clementi, E. Large molecules in carbon vapor. J. Am. Chem. Soc. 81, 4477–4485 (1959).
Google Scholar
-
Parasuk, V., Almlof, J. & Feyereisen, M. W. The [18] all-carbon molecule: cumulene or polyacetylene? J. Am. Chem. Soc. 113, 1049–1050 (1991).
Google Scholar
-
Torelli, T. & Mitas, L. Electron correlation in C4N+2 carbon rings: aromatic versus dimerized structures. Phys. Rev. Lett. 85, 1702–1705 (2000).
Google Scholar
-
Arulmozhiraja, S. & Ohno, T. CCSD calculations on C14, C18, and C22 carbon clusters. J. Chem. Phys. 128, 114301 (2008).
Google Scholar
-
Remya, K. & Suresh, C. H. Carbon rings: a DFT study on geometry, aromaticity, intermolecular carbon–carbon interactions and stability. RSC Adv. 6, 44261–44271 (2016).
Google Scholar
-
Kaiser, K. et al. An sp-hybridized molecular carbon allotrope, cyclo[18]carbon. Science 365, 1299–1301 (2019).
Google Scholar
-
Scriven, L. M. et al. Synthesis of cyclo[18]carbon via debromination of C18Br6. J. Am. Chem. Soc. 142, 12921–12924 (2020).
Google Scholar
-
Gao, Y. et al. On-surface synthesis of a doubly anti-aromatic carbon allotrope. Nature https://doi.org/10.1038/s41586-023-06566-8 (2023).
-
Baryshnikov, G. V., Valiev, R. R., Kuklin, A. V., Sundholm, D. & Agren, H. Cyclo[18]carbon: insight into electronic structure, aromaticity, and surface coupling. J. Phys. Chem. Lett. 10, 6701–6705 (2019).
Google Scholar
-
Baryshnikov, G. V. et al. Aromaticity of even-number cyclo[n]carbons (n = 6–100). J. Phys. Chem. A 124, 10849–10855 (2020).
Google Scholar
-
Charistos, N. D. & Muñoz-Castro, A. Induced magnetic field in sp-hybridized carbon rings: analysis of double aromaticity and antiaromaticity in cyclo[2N]carbon allotropes. Phys. Chem. Chem. Phys. 22, 9240–9249 (2020).
Google Scholar
-
Baryshnikov, G. V. et al. Odd-number cyclo[n]carbons sustaining alternating aromaticity. J. Phys. Chem. A 126, 2445–2452 (2022).
Google Scholar
-
Brémond, E., Pérez-Jiménez, A. J., Adamo, C. & Sancho-García, J. C. Stability of the polyynic form of C18, C22, C26, and C30 nanorings: a challenge tackled by range-separated double-hybrid density functionals. Phys. Chem. Chem. Phys. 24, 4515–4525 (2022).
Google Scholar
-
Li, M. et al. Potential molecular semiconductor devices: cyclo-Cn (n = 10 and 14) with higher stabilities and aromaticities than acknowledged cyclo-C18. Phys. Chem. Chem. Phys. 22, 4823–4831 (2020).
Google Scholar
-
Hoffmann, R. Extended Hückel theory―V: cumulenes, polyenes, polyacetylenes and Cn. Tetrahedron 22, 521–538 (1966).
Google Scholar
-
Liang, C. & Schaefer, H. F. III Carbon clusters: the structure of C10 studied with configuration interaction methods. J. Chem. Phys. 93, 8844–8849 (1990).
Google Scholar
-
Hutter, J., Lüthi, H. P. & Diederich, F. Structures and vibrational frequencies of the carbon molecules C2–C18 calculated by density functional theory. J. Am. Chem. Soc. 116, 750–756 (1994).
Google Scholar
-
Watts, J. D. & Bartlett, R. J. The nature of monocyclic C10. A theoretical investigation using coupled-cluster methods. Chem. Phys. Lett. 190, 19–24 (1992).
Google Scholar
-
Gross, L., Mohn, F., Moll, N., Liljeroth, P. & Meyer, G. The chemical structure of a molecule resolved by atomic force microscopy. Science 325, 1110–1114 (2009).
Google Scholar
-
Gross, L. et al. Bond-order discrimination by atomic force microscopy. Science 337, 1326–1329 (2012).
Google Scholar
-
Pavliček, N. et al. On-surface generation and imaging of arynes by atomic force microscopy. Nat. Chem. 7, 623–628 (2015).
Google Scholar
-
Pavliček, N. et al. Polyyne formation via skeletal rearrangement induced by atomic manipulation. Nat. Chem. 10, 853–858 (2018).
Google Scholar
-
Sun, Q. et al. On-surface formation of cumulene by dehalogenative homocoupling of alkenyl gem-dibromides. Angew. Chem. Int. Ed. Engl. 56, 12165–12169 (2017).
Google Scholar
-
Jones, R. R. & Bergman, R. G. p-Benzyne. Generation as an intermediate in a thermal isomerization reaction and trapping evidence for the 1,4-benzenediyl structure. J. Am. Chem. Soc. 94, 660–661 (1972).
Google Scholar
-
Lifshitz, C., Peres, T., Kababia, S. & Agranat, I. C10+· and C7+· carbon cluster ions from overcrowded octachloropentafulvalene and octachloronaphthalene. Int. J. Mass Spectrom. Ion Processes 82, 193–204 (1988).
Google Scholar
-
Lifshitz, C., Peres, T. & Agranat, I. Properties of carbon cluster ions, Cn+·, formed by dissociative ionization. Int. J. Mass Spectrom. Ion Processes 93, 149–163 (1989).
Google Scholar
-
Schuler, B. et al. Reversible Bergman cyclization by atomic manipulation. Nat. Chem. 8, 220–224 (2016).
Google Scholar
-
Albrecht, F. et al. Selectivity in single-molecule reactions by tip-induced redox chemistry. Science 377, 298–301 (2022).
Google Scholar
-
Suresh, R. et al. Cyclo[18]carbon formation from C18Br6 and C18(CO)6 precursors. J. Phys. Chem. Lett. 13, 10318–10325 (2022).
Google Scholar
-
Dobrowolski, M. A., Cyranski, M. K. & Wrobel, Z. Cyclic π-electron delocalization in non-planar linear acenes. Phys. Chem. Chem. Phys. 18, 11813–11820 (2016).
Google Scholar
-
Giessibl, F. J. High-speed force sensor for force microscopy and profilometry utilizing a quartz tuning fork. Appl. Phys. Lett. 73, 3956–3958 (1998).
Google Scholar
-
Albrecht, T. R., Grütter, P., Horne, D. & Rugar, D. Frequency modulation detection using high-Q cantilevers for enhanced force microscope sensitivity. J. Appl. Phys. 69, 668–673 (1991).
Google Scholar
-
Frisch, M. J. et al. Gaussian 16 Rev. C.01 (Gaussian, 2016).
-
Chai, J. D. & Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys. Chem. Chem. Phys. 10, 6615–6620 (2008).
Google Scholar
-
Hehre, W. J. Ab initio molecular orbital theory. Acc. Chem. Res. 9, 399–406 (1976).
Google Scholar
-
Lu, T. & Chen, F. Multiwfn: a multifunctional wavefunction analyzer. J. Comput. Chem. 33, 580–592 (2012).
Google Scholar
-
Humphrey, W., Dalke, A. & Schulten, K. VMD: visual molecular dynamics. J. Mol. Graph. 14, 33–38 (1996).
Google Scholar
-
Hapala, P. et al. Mechanism of high-resolution STM/AFM imaging with functionalized tips. Phys. Rev. B 90, 085421 (2014).
Google Scholar
-
Kresse, G. & Hafner, J. Ab initio molecular dynamics for open-shell transition metals. Phys. Rev. B 48, 13115–13118 (1993).
Google Scholar
-
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Google Scholar
-
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Google Scholar
-
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Google Scholar
-
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Google Scholar
-
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H–Pu. J. Chem. Phys. 132, 154104 (2010).
Google Scholar
-
Liu, Z., Lu, T. & Chen, Q. An sp-hybridized all-carboatomic ring, cyclo[18]carbon: bonding character, electron delocalization, and aromaticity. Carbon 165, 468–475 (2020).
Google Scholar
-
Lu, T. & Chen, Q. Interaction region indicator: a simple real space function clearly revealing both chemical bonds and weak interactions. Chem. Methods 1, 231–239 (2021).
Google Scholar
-
Johnson, E. R. et al. Revealing noncovalent interactions. J. Am. Chem. Soc. 132, 6498–6506 (2010).
Google Scholar
Acknowledgements
We acknowledge financial support from the National Natural Science Foundation of China (22125203).
Author information
Authors and Affiliations
Contributions
W.X. conceived the research. L.S., W.G. and F.K. performed the STM/AFM experiments and carried out the DFT calculations. W.Z. synthesized the C14Cl10 molecules. All authors contributed to writing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Glib Baryshnikov and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 The bond lengths, Mayer bond orders and bond angles in a cyclo[10]carbon.
Calculations were conducted at the ωB97XD/6-311 + + G(d,p) level. The bond lengths and bond order in C10 are all nearly the same, indicating the structure of consecutive carbon-carbon double bonds, i.e., cumulenic structure (BLA = 0).
Extended Data Fig. 2 Real-space function analysis of cyclo[10]carbon.
(a to d) Localized orbital locator calculated based on in-plane π MOs (LOL-πin) and out-plane π MOs (LOL-πout). (a) and (c) correspond to isosurface maps of LOL-π = 0.4. (b) shows LOL-πin in the ring plane, and (d) shows LOL-πout above 1 Bohr of the ring plane. (e) Interaction region indicator (IRI) isosurface and (f) color-filled map of C10 showing the homogeneous covalent interactions in carbon-carbon bonds. (g) Standard coloring method and chemical explanation of sign(λ2)ρ on interaction region indicator (IRI) isosurfaces.
Extended Data Fig. 3 STM images of the C10Cl8 precursor and the product cyclo[10]carbon.
(a) C10Cl8 molecules separately adsorbed on bilayer NaCl/Au(111) surface. A single CO molecule appeared as a small depression. (b) Close-up STM image of single C10Cl8 molecules on NaCl. (c) Close-up STM image of C10. Scanning condition: (a) I = 1 pA, V = 0.3 V. (b) I = 2 pA, V = 0.3 V. (c) I = 0.5 pA, V = 0.3 V.
Extended Data Fig. 4 Other intermediates observed during manipulation.
(a-c) C10Cl6, (d-f) C10Cl1. The Laplace-filtered AFM images are also shown. Reference set point of Δz: I = 0.5 pA, V = 0.3 V for (b), I = 1 pA, V = 0.3 V for (e). The double bonds indicated by blue and black in (d)represent two different bond lengths within the structure, respectively.
Extended Data Fig. 5 DFT relaxed C10 and C14 structures on NaCl surface.
(a, b) C10 on Cl-top site. (c, d) C10 on Na-top site. (e, f) C14 on Cl-top site. (g, h) C14 on Na-top site.
Extended Data Fig. 6 AFM images of C10 acquired at the oscillation amplitude A = 50 pm.
We tested the effect of different amplitude on the AFM imaging of C10. The AFM image (a, b) and AFM simulations (c, d) were carried out at the oscillation amplitude A = 50 pm. The Laplace-filtered AFM images of (a) and (b) are also shown in (e) and (f). Reference set point of Δz: I = 0.5 pA, V = 0.3 V. The scale bar in (a) applies to all experimental, simulated and Laplace-filtered AFM images.
Extended Data Fig. 7 The bond lengths, Mayer bond orders and bond angles in a cyclo[14]carbon.
Calculations were conducted at the ωB97XD/6-311 + + G(d,p) level, revealing a small bond length alternation (BLA = 0.05 Å) and bond angle alternation (BAA = 25.3°) within C14. The double bonds indicated by blue and black in C14 represent two different bond lengths within the structure, respectively.
Extended Data Fig. 8 The bond lengths, Mayer bond orders and bond angles in a cyclo[18]carbon.
Calculations were conducted at the ωB97XD/6-311 + + G(d,p) level, revealing a bond length alternation (BLA = 0.12 Å) within C18.
Extended Data Fig. 9 STM images of the C14Cl10 precursor and the product cyclo[14]carbon.
(a) C14Cl10 and CO molecules separately adsorbed on a bilayer NaCl/Au(111) surface. (b, c) STM and AFM images of an individual C14Cl10 molecule. (d) Spectra of frequency shift (Δf) as a function of tip height (Δz). The red and blue spectra were taken at the topmost Cl atom of the C14Cl10 molecule, and the Cl atom at the first layer of NaCl surface, respectively. Inset: schematics of the CO-tip approaching processes. The distance between two topmost Cl atoms of C14Cl10 molecule extracted from the AFM image and experimental absolute height extracted from the spectra reasonably agree with the theoretical values. (e) Close-up STM image of C14. Scanning condition: I = 1 pA, V = 0.3 V for (a); I = 0.5 pA, V = 0.3 V for (b); I = 1 pA, V = 0.3 V for (e). Reference set point of Δz for (c): I = 0.5 pA, V = 0.3 V.
Extended Data Fig. 10 AFM simulations of C14 with varying BLAs.
(a–h) A series of AFM simulations of C14 with varying BLAs from cumulenic to intermediate to polyynic structures at decreasing tip-sample distances from left to right (i.e., BLA = 0 Å, 0.03 Å, 0.05 Å, 0.07 Å, 0.09 Å, 0.11 Å, 0.13 Å, 0.15 Å) followed by the method developed in ref. 13. The simulated AFM images within blue and red boxes are assigned to cumulene-like and polyynic structures, respectively. All atomic coordinates keep the BAA as 25.3°. The double bonds indicated by blue and black in (b), (c) and (d) represent two different bond lengths within the structures, respectively. The scale bar in (a) applies to all simulated AFM images.
Extended Data Fig. 11 The bond lengths in the C14Cl4 and C14Cl1 intermediates.
The bond lengths of a ten-membered carbon ring in the C14Cl4 intermediate (a) and a fourteen-membered carbon ring in the C14Cl1 intermediate (b) are listed. Calculations were conducted at the ωB97XD/6-311 + + G(d,p) level. The double bonds indicated by blue and black in C14Cl1 represent two different bond lengths within the structure, respectively.
Extended Data Fig. 12 Other intermediates and side reaction products observed during manipulations.
(a–c) C14Cl8, (d-f) C14Cl3, (g–i) C14Cl5, (j–l) C14Cl6. The Laplace-filtered AFM images are also shown. Reference set point of Δz: I = 0.5 pA, V = 0.3 V for (b) and (e), I = 1 pA, V = 0.3 V for (h), I = 0.2 pA, V = 0.3 V for (k). The scale bar in (b) applies to all experimental and Laplace-filtered AFM images.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and Permissions
About this article
Cite this article
Sun, L., Zheng, W., Gao, W. et al. On-surface synthesis of aromatic cyclo[10]carbon and cyclo[14]carbon.
Nature 623, 972–976 (2023). https://doi.org/10.1038/s41586-023-06741-x
-
Received: 22 February 2023
-
Accepted: 11 October 2023
-
Published: 29 November 2023
-
Issue Date: 30 November 2023
-
DOI: https://doi.org/10.1038/s41586-023-06741-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.