Abstract
The use of real-time magnetic resonance imaging (rt-MRI) of speech is increasing in clinical practice and speech science research. Analysis of such images often requires segmentation of articulators and the vocal tract, and the community is turning to deep-learning-based methods to perform this segmentation. While there are publicly available rt-MRI datasets of speech, these do not include ground-truth (GT) segmentations, a key requirement for the development of deep-learning-based segmentation methods. To begin to address this barrier, this work presents rt-MRI speech datasets of five healthy adult volunteers with corresponding GT segmentations and velopharyngeal closure patterns. The images were acquired using standard clinical MRI scanners, coils and sequences to facilitate acquisition of similar images in other centres. The datasets include manually created GT segmentations of six anatomical features including the tongue, soft palate and vocal tract. In addition, this work makes code and instructions to implement a current state-of-the-art deep-learning-based method to segment rt-MRI speech datasets publicly available, thus providing the community and others with a starting point for developing such methods.
Background & Summary
Use of real-time magnetic resonance imaging (rt-MRI) to visualise articulators and the vocal tract during speech is increasing in both research and clinical settings1,2,3,4,5,6,7,8,9,10,11. This increase is a result of the development of real-time MRI techniques with relatively high spatio-temporal resolutions and the unique ability of MRI to non-invasively acquire images of any view without using ionising radiation.
Typically, in real-time speech MRI, series of two-dimensional (2D) images of a midsagittal slice of the vocal tract are acquired1,2,3,4,5,6,7,8,9,10,11. State-of-the-art real-time speech MRI techniques can acquire such images at spatial resolutions of 2.4 × 2.4 mm2 or higher at temporal resolutions of 0.02 s or higher12,13,14. However, these techniques require highly specialised equipment and software, namely custom receive coils13,14 and/or specialised pulse sequences and image reconstruction methods12,13,14 that are not widely available, particularly in clinical settings. Real-time speech MRI techniques that only require more widely available standard equipment and software have been developed15,16,17,18. While these techniques image at lower spatio-temporal resolutions than state-of-the-art ones, the resolutions are nevertheless sufficient to capture the global motion of the main articulators during speech2.
To widen access to real-time speech MRI data and therefore stimulate research in the field, several speech MRI datasets that include series of 2D real-time images of a midsagittal slice of the vocal tract have been made publicly available3,5,6,7,8,9,10,11. Most of these datasets include image series of English5,6,7 or French8,9 speakers performing phonologically comprehensive speech tasks (i.e. speech tasks designed to include most phonemes in a wide range of contexts). The other datasets include image series of English speakers producing emotional speech10, repeating several speech tasks consisting of vowel-consonant-vowel sequences11, and imitating unfamiliar speech sounds3.
There is increasing interest in extracting quantitative information from 2D midsagittal MR images of the vocal tract18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33. In particular, there is interest in measuring the size, shape and motion of the vocal tract18,19,22,23,24,25,26,27,28,29,30,31,32 and articulators such as the soft palate20,33,34,35,36,37. To avoid the burden of manual measurements, methods to (semi-)automatically measure the size and shape of the vocal tract have been developed38,39,40,41,42,43,44,45,46 and methods to automatically measure the size, shape and motion of the soft palate are beginning to be developed33,47,48,49,50. Consistent with trends in other image analysis fields, most of the recently developed methods utilise convolutional neural networks (CNNs) and are therefore deep learning based42,43,44,45,46,47,48,49,50.
Deep-learning-based methods are achieving state-of-the-art performance in a wide range of image analysis fields including medical image analysis51,52,53. However, a requirement for the development of such methods is ground-truth (GT) segmentations as well as images. These GT segmentations are manually created, a process that is time-consuming and, particularly for biomedical images, requires input by specialists. While GT segmentations for 2D midsagittal MR images of the vocal tract have been created46,47,48,49,50, none are currently publicly available. The public availability of image sets with corresponding GT segmentations has been found to stimulate the development of state-of-the-art image analysis methods54,55,56.
This work makes two main contributions to the literature. First, by making real-time speech MRI datasets with corresponding GT segmentations publicly available, it begins to address a major barrier to the development of deep-learning-based speech MR image analysis methods. Second, by making code and instructions to implement a current state-of-the-art deep-learning-based speech MR image analysis method47 publicly available, this work provides the speech MRI community and others with a starting point for the development of such methods. Although, the MRI data made available has been previously used in published work;47,50 neither data nor segmentations had been published. Since the previous work was published, the GT segmentations have been revised. In particular, the boundary of the soft and hard palate is defined using a radiological interpretation (in line with the anterior wall of the sphenoid sinus) as opposed to a tissue basis as the soft and hard palate overlap. The manuscript also provides image acquisition and segmentation details should the reader wish to increase the size of the dataset. The main intention of these contributions is to facilitate and stimulate the development of novel state-of-the-art speech MR image analysis methods.
Methods
Subjects
Following approval by the Health Research Authority (HRA) and with support from the Joint Research Management Office (JRMO), five healthy adult volunteers (two females, three males; age range 24–28 years) participated in the study after providing informed consent to publish the data, in accordance with ethics committee requirements (LREC 22/PR/0058). The volunteers were fluent English speakers and had no history of speech and language disorders. The provided data is fully anonymised with no personal information remaining.
Image acquisition
Each volunteer was imaged in a supine position using a 3.0 T TX Achieva MRI scanner and a 16-channel neurovascular coil (both Philips Healthcare, Best, Netherlands, software release 3.2) while they performed the following speech task a single time: counting from 1 to 10 in English. Images of a 10 mm thick mid-sagittal slice of the head were acquired using a steady state free procession (SSFP) pulse sequence based on the sequence identified by Scott et al.15 as being optimal for vocal tract image quality. Example images are shown in Fig. 1A. Imaging parameters are listed in Table 1. The acquired matrix size and in-plane pixel size were 120 × 93 and 2.5 × 2.45 mm2 respectively. However, k-space data were zero padded to a matrix size of 256 × 256 by the scanner before being reconstructed, resulting in a reconstructed in-plane pixel size of 1.17 × 1.17 mm2. To maximise the signal-to-noise ratio in the images, partial Fourier was not used. One image series was acquired per volunteer at a temporal resolution of 0.1 s. The volunteers were instructed to perform the speech task at a rate which they considered to be normal. Some performed the task faster than others and consequently not all series had the same number of images. The series had 105, 71, 71, 78 and 67 images each (392 images in total). Each series required a total scan time of 10.5, 7.1, 7.1, 7.8 and 6.7 s respectively. The process to identify the midsagittal plane was as follows. First, a localiser scan was performed that acquired series of 2D images of three perpendicular (approximately the axial, coronal and sagittal planes). Second, the images of the approximate sagittal planes were visually inspected and the plane of the image that most closely resembled a midsagittal plane was selected. Third, this plane was manually adjusted so that it passed through the nasal septum and between the two hemispheres of the brain in the images of the approximate axial planes.

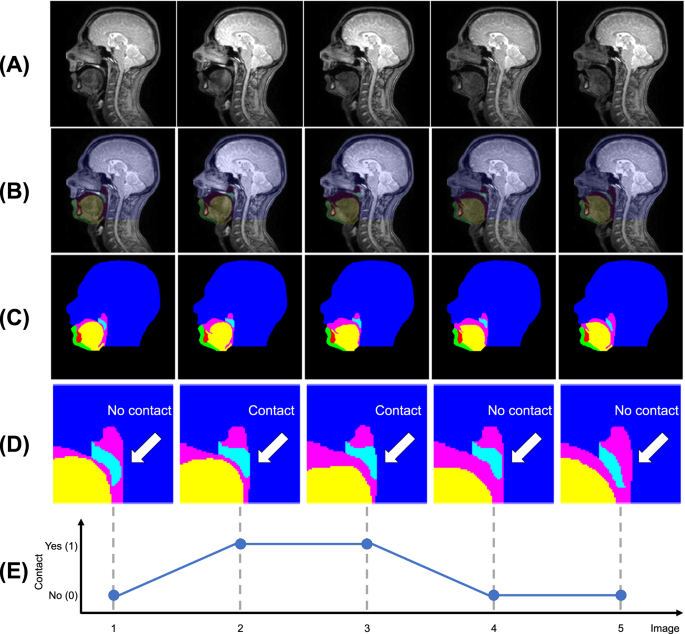
Five consecutive images from one of the magnetic resonance image series (A), corresponding ground-truth (GT) segmentations overlaid on the images (B), GT segmentations only (C), GT segmentations cropped around the soft palate with labels indicating if there is contact between the soft palate and posterior pharyngeal wall (D), and a line chart indicating if there is contact (Yes) or not (No) between the soft palate and posterior pharyngeal wall in each image in the series (E). The GT segmentations are of the head (dark blue), soft palate (light blue), jaw (green), tongue (yellow), vocal tract (pink) and tooth space (red) classes.
Velopharyngeal closure identification
Each image was visually inspected and labelled as either showing contact between the soft palate and posterior pharyngeal wall or not showing contact. Example images showing contact and no contact are shown in Fig. 1A. Line charts of the labels of each image series were created (example labels are shown in Fig. 1D) and visually inspected to determine the number of velopharyngeal closures shown in each series. In these charts, each peak represents a velopharyngeal closure, as consecutive images where the soft palate is in contact with the posterior pharyngeal wall show a single velopharyngeal closure. It can be challenging to determine if a 2D real-time MR image shows contact between the soft palate and posterior pharyngeal wall, especially if the soft palate is close to the posterior pharyngeal wall or if there is fluid surrounding the tissues, which can conceal tissue boundaries. To reduce the subjectivity of the labels, each image was independently labelled by four MRI Physicists. Raters one to four respectively had four, ten, two and one years of speech MRI experience. All the images were labelled again one month later by rater one. Intra- and inter-rater agreement was assessed by comparing the labels and the number of velopharyngeal closures determined from these labels. In cases where one rater disagreed with the others, the majority label was considered to be the GT label. In cases where only two raters agreed, raters one and two (those with the most speech MRI experience) jointly inspected the images and then reached a consensus on the labels for these images, similarly to how speech and language therapists jointly inspect videofluoroscopy speech image series in clinical practice in the United Kingdom. The consensus labels were used as the GT labels and the GT number of velopharyngeal closures was determined from these. Line charts of the GT labels are shown in Fig. 2.

Ground-truth labels of the five image series. Each line chart represents a different series and has a different x-axis. Each peak in a line chart indicates a velopharyngeal closure.
Ground-truth segmentation creation
GT segmentations were created by manually labelling pixels in each of the images. The segmentations consisted of six classes, each made up of one or more anatomical features. There was no overlap between classes: a pixel could not belong to more than one class. For conciseness, the classes were named as follows: head, soft palate, jaw, tongue, vocal tract and tooth space. However, the names of the head, jaw and tongue classes are simplifications. The head class consisted of all anatomical features superior to or posterior to the vocal tract. It therefore included the upper lip, hard palate, brain, skull, posterior pharyngeal wall, and neck. The jaw class consisted of the lower lips, the soft tissue anterior to and inferior to the mandible and the soft tissue inferior to the tongue. The tongue class included the epiglottis and the hyoid bone. Pixels not labelled as belonging to one of the classes were considered to belong to the background. Example GT segmentations are shown in Figs. 2 and 3B. The reasons for including the classes in the GT segmentations are given in Table 2.
Wherever possible, the boundaries of the classes were chosen to be clear anatomical boundaries in order to minimise the subjectivity of the GT segmentations. Apart from the tooth space class, the majority of the class boundaries were easily distinguishable air-tissue boundaries. However, there were no clear anatomical boundaries for some sections of the class boundaries. Instead, the following artificial boundaries were devised for these sections. The two main goals when devising these boundaries were firstly to include only relevant anatomical features and secondly to minimise the subjectivity of the boundaries.
The inferior boundary of the head class in the neck was defined as the horizontal line parallel to the inferior surface of the intervertebral disc between cervical vertebrae C3 and C4 (see dark blue arrows in Fig. 3). This choice was made to exclude the inferior section of the neck in the head class as this section was not required for the desired analyses and would have otherwise increased the imbalance between the number of pixels in the head class and the other classes.

An image cropped to only show the vocal tract (A) with ground-truth segmentations overlaid (B). The dark blue arrows point to the inferior surface of the intervertebral disc between cervical vertebrae C3 and C4. The light blue arrows point to anterior wall of the sphenoid sinus. The dotted green arrows point to the anterior edge of the hyoid bone, while the solid green arrows point to where the neck meets the jaw. The yellow arrows point to the inferior boundary of the tongue class in the neck, while the pink arrows point to the inferior boundary of the vocal tract class.
The boundary where the soft palate connects to the head class was defined to be in line with the anterior wall of the sphenoid sinus, and the boundary edge is perpendicular to the dark line that follows the edge of the hard palate (see light blue arrows in Fig. 3). The posterior boundary of the jaw class was defined as the anterior edge of the hyoid bone (see dotted green arrows in Fig. 3), while the inferior boundary of the jaw class in the neck was defined as the horizontal line intersecting the point where the jaw meets the neck (see solid green arrows in Fig. 3). The inferior boundary of the vocal tract class was defined in the same way as that of the head class (see pink arrows in Fig. 3), and the inferior boundary of the tongue class in the neck was defined in the same way as that of the jaw class in the neck (see yellow arrows in Fig. 3).
GT segmentations were created by the MRI Physicist with four years of speech MRI experience, using bespoke software developed in house and implemented in MATLAB R2019b (MathWorks, Natick, MA). GT segmentations were consistent with the GT label for the images: segmentations of the soft palate and posterior pharyngeal wall (part of the head class) were in contact for images labelled as showing contact and not in contact otherwise.
Data Records
The datasets are available on Zenodo57 (version 2) and consist of the five 2D real-time MR image series, GT segmentations and GT contact labels described in this article. The directory containing the datasets is structured in the way shown in Fig. 4. Images are contained in the MRI_SSFP_10fps folder. Within this folder, each subfolder contains the images of a different volunteer. Each image is saved as a separate anonymised DICOM file with name image_N.dcm. GT contact labels are saved in velopharyngeal_closure.xslx. The labels of each volunteer are saved in different sheets. The spreadsheet row corresponds to the image number (i.e. the label in row 1 is the label for image 1). A label of 1 indicates contact while 0 indicates no contact. GT segmentations are contained in the GT_Segmentations folder. Within this folder, each subfolder contains the GT segmentations of a different volunteer. Each GT segmentation is saved as a separate MAT file with name mask_N.mat. In each MAT file, pixels with the following values correspond to the following class:
-
0 = background
-
1 = head
-
2 = soft palate
-
3 = jaw
-
4 = tongue
-
5 = vocal tract
-
6 = tooth space

The structure of the directory containing the datasets on Zenodo.
Technical Validation
Imaging
To increase the likelihood of acquiring images with a good image quality, a sequence based on the one identified by Scott et al.15 as being optimal in terms of image quality for 2D real-time vocal tract imaging at 3.0 T was used in this work. Nevertheless, before they were manually segmented, all the images were visually inspected by the MRI Physicists with four and ten years of speech MRI experience, to verify that air-tissue boundaries between the vocal tract and articulators were clearly visible and that no artefacts obscured the articulators in the images.
Velopharyngeal closure analysis
The subjectivity of the GT closure labels was investigated by assessing the intra- and inter-rater agreement in the labels. As shown in Fig. 5, there was intra-rater agreement in the labels for 98.2% (385 of 392) images and in all 30 velopharyngeal closures. In three image series, intra-rater agreement in the labels was 100% (220 of 220) images, while in the other two image series intra-rater agreement in the labels was 97.0% (65 of 67) and 95.2% (100 of 105) images respectively. All label differences were for images at the start or end of a velopharyngeal closure, where the soft palate is close to or in contact with the posterior pharyngeal wall. Such discrepancies affected the durations of velopharyngeal closures but not the number of velopharyngeal closures.

Intra- and inter-rater agreement in the labels of the 392 images (A) and in the velopharyngeal closures (B).
There was complete inter-rater agreement in the labels of 357 of 392 (91.1%) images and in 25 of 30 (83.3%) velopharyngeal closures. All label differences were for images where the soft palate was close to or in contact with the posterior pharyngeal wall. In two image series, there was complete inter-rater agreement in all 12 velopharyngeal closures. In the other three image series, there was complete inter-rater agreement in 5 of 6 (83.3%), 3 of 4 (75.0%) and 5 of 8 (62.5%) velopharyngeal closures respectively. As shown in Fig. 5, raters one and two had the highest inter-rater agreement, with agreement in the labels of 384 of 392 (98.0%) images and in all 30 velopharyngeal closures. There was inter-rater agreement between at least three raters in the labels of 385 of 392 (98.2%) images and in all 30 velopharyngeal closures. Figure 6 shows images where inter-rater agreement in labels was low. In all five cases where there was inter-rater disagreement in a velopharyngeal closure, one of the raters considered there to be two closures instead of one.

Images cropped to only show the vocal tract (A) and soft palate (B) where only two out of four raters agreed on the label.
Ground-truth segmentation creation
The largest to smallest GT segmentation classes in terms of number of pixels are the head with a median of 23633 pixels per segmentation (ranging between 21093–21353 pixels), tongue with 2075 pixels (1936–2298), vocal tract with 1153 pixels (754–1560), lower jaw with 945 (827–1133), soft palate with 237 pixels (187–268) and tooth space with 160 pixels (104–187).
To enable investigation of intra-rater agreement and therefore uncertainty in the segmentations, the Physicist created GT segmentations again for seven (approximately 10%) randomly chosen images in each series. The agreement was quantified using two widely-used metrics in the medical image analysis community58: the Dice coefficient (DSC) and the general Hausdorff distance (HD). The median intra-rater agreement was 0.965 and 2.24 pixels respectively. As shown in Fig. 7, intra-rater agreement was highest for segmentations of the head class with a median DSC of 0.997 and a median HD of 2.8 pixels, while intra-rater agreement was lowest for segmentations of the tooth space and soft palate classes, with median DSC of 0.929 and 0.933, and a median HD of 1.41 and 1.41 pixels, respectively. Segmentations of the soft palate class had the largest range in DSCs, closely followed by segmentations of the tooth space.

Intra-rater agreement in the ground-truth segmentations, quantified using the Dice coefficient (A) and general Hausdorff Distance (B).
Usage Notes
The datasets described in this article can be used to develop methods to segment speech MR images. Code to train and evaluate such a method47 using the datasets is publicly available in the following GitHub repository: https://github.com/BartsMRIPhysics/Speech_MRI_2D_UNet (software licence: Apache-2.0). The datasets and the code provide the speech MRI community and others with a starting point for developing such methods. In conjunction with other datasets, the datasets described in this article could be used to develop methods to segment a broader range of speech images acquired using different imaging techniques. The datasets could also easily be modified to enable the development of methods to analyse air-tissue boundaries in speech images, such as methods38,39,40,41,42,43,44,45.
This dataset contains images of only five healthy volunteers all acquired using the same MRI scanner and sequence combination. Although the low number of subjects is a limitation, it was previously shown to be sufficient to develop and train segmentation networks47 or to inform registration50. Clear instructions for acquisition and segmentations are given for readers who wish to increase the size of the dataset.
Code availability
The code that accompanies this article is publicly available in the following GitHub repository: https://github.com/BartsMRIPhysics/Speech_MRI_2D_UNet (software licence: Apache version 2.0). The repository contains already trained versions of a state-of-the-art speech MR image segmentation method47 that are ready to use immediately. These versions were trained using the datasets described in this article. The repository also contains instructions and Python code to train and evaluate new versions of the method using the datasets described in this article. The code is designed to allow users to choose several important training parameters such as the training and validation dataset split, the number of epochs of training, the learning rate and the mini-batch size. In addition, the code is designed to be compatible with any dataset as long as it is organised and named in a specific way. The repository contains Python code to check that the datasets are not corrupted and are organised and named in the specific way required by the segmentation method, as well as Python code to perform the image pre-processing required by the method, namely normalising the images and saving the normalised images as MAT files.
References
-
Scott, A. D., Wylezinska, M., Birch, M. J., Miquel, M. E. & Speech, M. R. I. Morphology and function. Phys. Medica 30, 604–618 (2014).
Google Scholar
-
Lingala, S. G., Sutton, B. P., Miquel, M. E. & Nayak, K. S. Recommendations for real-time speech MRI. J. Magn. Reson. Imaging 43, 28–44 (2016).
Google Scholar
-
McGettigan, C., Miquel, M., Carey, D., Waters, S. & Kanber, E. Vocal Learning in Adulthood: Investigating the mechanisms of vocal imitation using MRI of the vocal tract and brain 2015–2018. UK Data Service https://doi.org/10.5255/UKDA-SN-853317 (2018).
-
Nayak, K. S., Lim, Y., Campbell-Washburn, A. E. & Steeden, J. Real-Time Magnetic Resonance Imaging. J. Magn. Reson. Imaging 55, 81–99 (2022).
Google Scholar
-
Narayanan, S. et al. Real-time magnetic resonance imaging and electromagnetic articulography database for speech production research (TC. ). J. Acoust. Soc. Am. 136, 1307–1311 (2014).
Google Scholar
-
Sorensen, T. et al. Database of volumetric and real-time vocal tract MRI for speech science. in INTERSPEECH 645–649, https://doi.org/10.21437/Interspeech.2017-608 (2017).
-
Lim, Y. et al. A multispeaker dataset of raw and reconstructed speech production real-time MRI video and 3D volumetric images. Sci. Data 8, 1–14 (2021).
Google Scholar
-
Douros, I. K. et al. A multimodal real-time MRI articulatory corpus of French for speech research. In Proc. Annu. Conf. of the Int. Speech Commun. Assoc. (INTERSPEECH) 1556–1560, https://doi.org/10.21437/Interspeech.2019-1700 (2019).
-
Isaieva, K. et al. Multimodal dataset of real-time 2D and static 3D MRI of healthy French speakers. Sci. Data 8, 1–9 (2021).
Google Scholar
-
Kim, J. et al. USC-EMO-MRI corpus: An emotional speech production database recorded by real-time magnetic resonance imaging. In Proc. of the 10th Int. Seminar on Speech Prod. (ISSP) 2014, 226–229 (2014).
-
Töger, J. et al. Test–retest repeatability of human speech biomarkers from static and real-time dynamic magnetic resonance imaging. J. Acoust. Soc. Am. 141, 3323–3336 (2017).
Google Scholar
-
Uecker, M. et al. Real-time MRI at a resolution of 20 ms. NMR Biomed. 23, 986–994 (2010).
Google Scholar
-
Lingala, S. G. et al. State-of-the-art MRI protocol for comprehensive assessment of vocal tract structure and function. In Proc. Annu. Conf. Int. Speech Commun. Assoc. (Interspeech) 475–479, https://doi.org/10.21437/Interspeech.2016-559 (2016).
-
Lingala, S. G. et al. A fast and flexible MRI system for the study of dynamic vocal tract shaping. Magn. Reson. Med. 77, 112–125 (2017).
Google Scholar
-
Scott, A. D., Boubertakh, R., Birch, M. J. & Miquel, M. E. Towards clinical assessment of velopharyngeal closure using MRI: evaluation of real-time MRI sequences at 1.5 and 3 T. Br. J. Radiol. 85, e1083–e1092 (2012).
Google Scholar
-
Freitas, A. C., Wylezinska, M., Birch, M. J., Petersen, S. E. & Miquel, M. E. Comparison of Cartesian and Non-Cartesian Real-Time MRI Sequences at 1.5T to Assess Velar Motion and Velopharyngeal Closure during Speech. PLoS One 11, e0153322 (2016).
Google Scholar
-
Freitas, A. C., Ruthven, M., Boubertakh, R. & Miquel, M. E. Real-time speech MRI: Commercial Cartesian and non-Cartesian sequences at 3T and feasibility of offline TGV reconstruction to visualise velopharyngeal motion. Phys. Medica 46, 96–103 (2018).
Google Scholar
-
Carey, D., Miquel, M. E., Evans, B. G., Adank, P. & McGettigan, C. Vocal Tract Images Reveal Neural Representations of Sensorimotor Transformation During Speech Imitation. Cereb. Cortex 33, 316–325 (2017).
-
Carignan, C., Shosted, R. K., Fu, M., Liang, Z. P. & Sutton, B. P. A real-time MRI investigation of the role of lingual and pharyngeal articulation in the production of the nasal vowel system of French. J. Phon. 50, 34–51 (2015).
Google Scholar
-
Arendt, C. T. et al. Comparison of contrast-enhanced videofluoroscopy to unenhanced dynamic MRI in minor patients following surgical correction of velopharyngeal dysfunction. Eur. Radiol. 31, 76–84 (2021).
Google Scholar
-
Perry, J. L. et al. Establishing a Clinical Protocol for Velopharyngeal MRI and Interpreting Imaging Findings. Cleft Palate-Craniofac. J. 10556656221141188, https://doi.org/10.1177/10556656221141188 (2022).
-
Hagedorn, C., Kim, J., Sinha, U., Goldstein, L. & Narayanan, S. S. Complexity of vocal tract shaping in glossectomy patients and typical speakers: A principal component analysis. J. Acoust. Soc. Am. 149, 4437–4449 (2021).
Google Scholar
-
Wiltshire, C. E. E., Chiew, M., Chesters, J., Healy, M. P. & Watkins, K. E. Speech Movement Variability in People Who Stutter: A Vocal Tract Magnetic Resonance Imaging Study. J. Speech, Lang. Hear. Res. 64, 2438–2452 (2021).
Google Scholar
-
Lu, Y., Wiltshire, C. E. E., Watkins, K. E., Chiew, M. & Goldstein, L. Characteristics of articulatory gestures in stuttered speech: A case study using real-time magnetic resonance imaging. J. Commun. Disord. 97, 106213 (2022).
Google Scholar
-
Belyk, M. & McGettigan, C. Real-time magnetic resonance imaging reveals distinct vocal tract configurations during spontaneous and volitional laughter. Philos. Trans. R. Soc. B Biol. Sci. 377, 20210511 (2022).
Google Scholar
-
Silva, S. & Teixeira, A. Quantitative systematic analysis of vocal tract data. Comput. Speech Lang. 36, 307–329 (2016).
Google Scholar
-
Ramanarayanan, V. et al. Analysis of speech production real-time MRI. Comput. Speech Lang. 52, 1–22 (2018).
Google Scholar
-
Kim, J., Toutios, A., Lee, S. & Narayanan, S. S. Vocal tract shaping of emotional speech. Comput. Speech Lang. 101100, https://doi.org/10.1016/j.csl.2020.101100 (2020).
-
Carignan, C. et al. Analyzing speech in both time and space: Generalized additive mixed models can uncover systematic patterns of variation in vocal tract shape in real-time MRI. Lab. Phonol. J. Assoc. Lab. Phonol. 11, 2 (2020).
Google Scholar
-
Leppävuori, M. et al. Characterizing Vocal Tract Dimensions in the Vocal Modes Using Magnetic Resonance Imaging. J. Voice 35, 804.e27–804.e42 (2021).
Google Scholar
-
Belyk, M., Waters, S., Kanber, E., Miquel, M. E. & McGettigan, C. Individual differences in vocal size exaggeration. Sci. Rep. 12, 1–12 (2022).
Google Scholar
-
Ikävalko, T. et al. Three Professional Singers’ Vocal Tract Dimensions in Operatic Singing, Kulning, and Edge—A Multiple Case Study Examining Loud Singing. J. Voice https://doi.org/10.1016/j.jvoice.2022.01.024 (2022).
-
Carignan, C. et al. Planting the seed for sound change: Evidence from real-time MRI of velum kinematics in German. Lang. (Baltim). 97, 333–364 (2021).
-
Seselgyte, R., Swan, M. C., Birch, M. J. & Kangesu, L. Velopharyngeal Incompetence in Children With 22q11.2 Deletion Syndrome: Velar and Pharyngeal Dimensions. J. Craniofac. Surg. 32, 578–580 (2021).
Google Scholar
-
Tian, W. & Redett, R. J. New velopharyngeal measurements at rest and during speech: Implications and applications. J. Craniofac. Surg. 20, 532–539 (2009).
Google Scholar
-
Tian, W. et al. Magnetic resonance imaging assessment of velopharyngeal motion in Chinese children after primary palatal repair. J. Craniofac. Surg. 21, 578–587 (2010).
Google Scholar
-
Tian, W. et al. Magnetic resonance imaging assessment of the velopharyngeal mechanism at rest and during speech in Chinese adults and children. J. Speech, Lang. Hear. Res. 53, 1595–1615 (2010).
Google Scholar
-
Bresch, E. & Narayanan, S. Region segmentation in the frequency domain applied to upper airway real-time magnetic resonance images. IEEE Trans. Med. Imaging 28, 323–338 (2009).
Google Scholar
-
Kim, J., Kumar, N., Lee, S. & Narayanan, S. Enhanced airway-tissue boundary segmentation for real-time magnetic resonance imaging data. In Proc. 10th Int. Seminar Speech Prod. (ISSP) 222–225 (2014).
-
Silva, S. & Teixeira, A. Unsupervised segmentation of the vocal tract from real-time MRI sequences. Comput. Speech Lang. 33, 25–46 (2015).
Google Scholar
-
Labrunie, M. et al. Automatic segmentation of speech articulators from real-time midsagittal MRI based on supervised learning. Speech Commun. 99, 27–46 (2018).
Google Scholar
-
Somandepalli, K., Toutios, A. & Narayanan, S. S. Semantic Edge Detection for Tracking Vocal Tract Air-tissue Boundaries in Real-time Magnetic Resonance Images. In INTERSPEECH 631–635 (2017).
-
Valliappan, C., Mannem, R. & Ghosh, P. K. Air-tissue boundary segmentation in real-time magnetic resonance imaging video using semantic segmentation with fully convolutional networks. In INTERSPEECH 3132–3136, https://doi.org/10.21437/Interspeech.2018-1939 (2018).
-
Valliappan, C., Kumar, A., Mannem, R., Karthik, G. & Ghosh, P. K. An improved air tissue boundary segmentation technique for real time magnetic resonance imaging video using SegNet. in IEEE Int. Conf. Acoust., Speech and Sign. Proc. 5921–5925 (2019).
-
Mannem, R. & Ghosh, P. K. Air-tissue boundary segmentation in real time magnetic resonance imaging video using a convolutional encoder-decoder network. in IEEE Int. Conf. Acoust., Speech and Sign. Proc. 5941–5945 (2019).
-
Erattakulangara, S. & Lingala, S. G. Airway segmentation in speech MRI using the U-net architecture. in IEEE Int. Symp. on Biomed. Imaging 1887–1890 (2020).
-
Ruthven, M., Miquel, M. E. & King, A. P. Deep-learning-based segmentation of the vocal tract and articulators in real-time magnetic resonance images of speech. Comput. Methods Programs Biomed. 198, 105814 (2021).
Google Scholar
-
Bonà, A. & Cavicchioli, M. Vocal tract segmentation of dynamic speech MRI images based on deep learning for neurodegenerative disease application. Master’s thesis, Politecnico di Milano (2021).
-
Ivanovska, T. et al. A deep cascaded segmentation of obstructive sleep apnea-relevant organs from sagittal spine MRI. Int. J. Comput. Assist. Radiol. Surg. 16, 579–588 (2021).
Google Scholar
-
Ruthven, M., Miquel, M. E. & King, A. P. A segmentation-informed deep learning framework to register dynamic two-dimensional magnetic resonance images of the vocal tract during speech. Biomed. Signal Process. Control 80, 104290 (2023).
Google Scholar
-
Litjens, G. et al. A survey on deep learning in medical image analysis. Med. Image Anal. 42, 60–88 (2017).
Google Scholar
-
Sermesant, M., Delingette, H., Cochet, H., Jaïs, P. & Ayache, N. Applications of artificial intelligence in cardiovascular imaging. Nat. Rev. Cardiol. 18, 600–609 (2021).
Google Scholar
-
Chen, X. et al. Recent advances and clinical applications of deep learning in medical image analysis. Med. Image Anal. 79, 102444 (2022).
Google Scholar
-
Heller, N. et al. The state of the art in kidney and kidney tumor segmentation in contrast-enhanced CT imaging: Results of the KiTS19 challenge. Med. Image Anal. 67, 101821 (2021).
Google Scholar
-
Campello, V. M. et al. Multi-Centre, Multi-Vendor and Multi-Disease Cardiac Segmentation: The MMs Challenge. IEEE Trans. Med. Imaging 40, 3543–3554 (2021).
Google Scholar
-
Antonelli, M. et al. The Medical Segmentation Decathlon. Nat. Commun. 13, 1–13 (2022).
Google Scholar
-
Ruthven, M., Peplinski, A. & Miquel, M. A multi-speaker dataset of real-time two-dimensional speech magnetic resonance images with articulator ground-truth segmentations (v2) [Dataset]. Zenodo. https://doi.org/10.5281/zenodo.10046815 (2023).
-
Reinke, A. et al. Common Limitations of Image Processing Metrics: A Picture Story. ArXiv:2104.05642 (2022).
Acknowledgements
The authors are grateful to Dr Thomas Campion (Consultant Radiologist, Barts Health NHS Trust) and Mr Loshan Kangesu (Consultant Plastic Surgeon, Mid Essex Hospital Services NHS Trust) for expert advice on how to best segment the different articulator groups. Matthieu Ruthven is funded by a Health Education England/National Institute for Health Research Clinical Doctoral Fellowship for this project and Agnieszka Peplinski by Barts Charity.
Author information
Authors and Affiliations
Contributions
Matthieu Ruthven was the main contributor to this work. He collected the data, created the ground-truth segmentations, labelled the velopharyngeal closures and prepared the manuscript. Agnieszka Peplinski helped to create the ground-truth segmentation consensus, label velopharyngeal closures and prepared the manuscript. David Adams labelled velopharyngeal closures. Andrew King supervised this work and gave guidance throughout. Marc Miquel supervised this work and gave guidance throughout, helped to create the ground-truth segmentation consensus, labelled velopharyngeal closures and helped to prepare the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
Reprints and Permissions
About this article
Cite this article
Ruthven, M., Peplinski, A.M., Adams, D.M. et al. Real-time speech MRI datasets with corresponding articulator ground-truth segmentations.
Sci Data 10, 860 (2023). https://doi.org/10.1038/s41597-023-02766-z
-
Received: 28 August 2023
-
Accepted: 20 November 2023
-
Published: 02 December 2023
-
DOI: https://doi.org/10.1038/s41597-023-02766-z