Abstract
Background/Objectives
Childhood obesity, particularly in girls, is linked to early puberty onset, heightening risks for adult-onset diseases. Addressing childhood obesity and precocious puberty is vital to mitigate societal burdens. Despite existing costly and invasive medical interventions, introducing lifestyle-based alternatives is essential. Our study investigates alternate-day fasting’s (ADF) impact on pubertal development in normal-weight and high-fat diet (HFD)-induced obese female mice.
Methods
Four groups of female mice were utilized, with dams initially fed control chow during and before pregnancy. Post-parturition, two groups continued on control chow, while two switched to an HFD. Offspring diets mirrored maternal exposure. One control and one HFD group were subjected to ADF. Morphometry and hormone analyses at various time points were performed.
Results
Our findings demonstrate that ADF in normal-weight mice led to reduced body length, weight, uterine, and ovarian weights, accompanied by delayed puberty and lower levels of sex hormones and growth hormone (GH). Remarkably, GH treatment effectively prevented ADF-induced growth reduction but did not prevent delayed puberty. Conversely, an HFD increased body length, induced obesity and precocious puberty, and altered sex hormones and leptin levels, which were counteracted by ADF regimen. Our data indicate ADF’s potential in managing childhood obesity and precocious puberty.
Conclusions
ADF reduced GH and sex hormone levels, contributing to reduced growth and delayed puberty, respectively. Therefore, parents of normal-weight children should be cautious about prolonged overnight fasting. ADF prevented HFD-induced obesity and precocious puberty, offering an alternative to medical approaches; nevertheless, further studies are needed for translation into clinical practice.
Introduction
Precocious puberty stands as a prominent global issue in the 21st century, with contributing factors such as overnutrition and childhood overweight leading to its prevalence. Both childhood obesity and precocious puberty are risk factors for health issues in childhood and adulthood [1, 2]. Precocious puberty occurs due to the early activation of the hypothalamic-pituitary-gonadal axis, leading to the premature development of secondary sexual characteristics [3]. We recently defined precocious puberty in the Chinese population, setting the onset before 7.5 and 9 years of age for girls and boys, respectively [4]. Additionally, childhood overweight and obesity are associated with an increased risk of precocious puberty in China [4]. Similarly, our animal studies demonstrated that postnatal overfeeding of an HFD induces both obesity and precocious puberty in mice [5, 6]. While perinatal undernutrition reduces body weight and delays puberty [7], these findings underscore the crucial role of perinatal nutrition in regulating pubertal development, emphasizing that an imbalanced diet during this critical window results in earlier or delayed pubertal development.
We have previously reported that the prevalence of both childhood obesity and precocious puberty is increasing in China [4, 8], necessitating effective control measures. Traditional treatment protocols, although effective [9,10,11,12,13], often involve invasive, costly approaches with potential side effects. For instance, Gonadotropin-releasing hormone agonist treatment efficiently controls precocious puberty but comes with various health risks [14,15,16,17,18,19]. Hence, there is a critical need to introduce an alternative, safe method for managing both childhood obesity and precocious puberty. Intermittent fasting, known to inhibit the hypothalamic-pituitary-gonadal axis in rats [20], and improve body weight and fat in obese subjects [21,22,23,24,25], is hypothesized to be effective in controlling both childhood obesity and precocious puberty. Intermittent fasting involves eating and restricting feeding during specific times of the day or week; for details, interested readers can refer to [24]. In our study, we applied ADF, a type of intermittent fasting, after lactation (postnatal day 21) and observed reduced body growth and delayed puberty onset in mice fed on control chow. Intriguingly, ADF prevented accelerated body growth and precocious puberty in mice feeding on HFD. This study presents the pioneering role of ADF in controlling HFD-induced childhood obesity and accelerated pubertal development, with the potential to translate into clinical practice.
Materials and methods
Animals
C57BL/6J mice were employed for this study. The mice were purchased from GEMPHARMATECH in Shanghai, China, and housed in the animal facility at Zhejiang University. Standard conditions were maintained for air quality, humidity, and temperature, following a 12 h light:12 h dark cycle (lights on at 6:00 A.M.). Mice had ad libitum access to food and water throughout the study. Breeding pairs were established at 12 weeks of age, with daily checks on pregnant dams. The discovery of new pups marked postnatal day 1 (P1), and only female pups were included in the experiment. This study adhered to the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and received approval from the Animal Advisory Committee at Zhejiang University.
Diets
Two diets were employed in the study: an HFD (60 kcal% fat, 20 kcal% protein, and 20 kcal% carbohydrates, D12492, Research Diets) and standard control chow (15 kcal% fat, 24 kcal% protein, and 61 kcal% carbohydrates, 1010097 Jiangsu Xietong Pharmaceutical Bio-engineering Co., Ltd).
Experimental design
The dams were maintained on a standard control chow diet before and during pregnancy. At parturition, dams were exposed to either control chow or HFD. Post-weaning at P21, the offspring continued their respective diets. ADF was implemented following the protocol described by [20, 24] with minor adjustments. The animals experienced 24 h of fasting starting at 5 P.M., or an 18 h overnight fast from 5 P.M. to 11 A.M. on alternate days. Offspring fed on control chow or HFD without ADF were designated as the control group (Cntrl) or HFD group, respectively. It is important to note that for the HFD group with ADF, food removal occurred at 5 P.M. for a regular 24 h fasting cycle; however, for experiments requiring blood collection at 11 A.M. on P34, food removal was adjusted to 11 A.M. on the day before the scheduled blood collection. On average, vaginal opening (VO) occurs at approximately P33 in control mice, whereas 18 h ADF delays it, typically around P40. Consequently, two specific time points, P34 and P40, were selected for a range of analyses in mice fed with chow. The litter size ranged from 6 to 7 pups. We measured various parameters, and the number of mice used for each parameter varied. The number of mice per group ranged from 6 to 20, depending on the specific parameter being analyzed. Each data point in the bar graphs corresponds to an individual mouse, so the number of data points within each graph represents the number of mice used for the analyses. Mice that were weak, sick, or had haemolyzed samples were excluded from the experiment. The experimental design is illustrated in Supplementary Fig. 1.
Measurement of body weight, body length, and puberty onset assessment
Body weight and body length, serving as indices for obesity and overall body growth, were measured. Puberty onset was assessed using vaginal canalization, a recognized marker in rodent studies [5, 6, 26,27,28,29]. Pups were consistently monitored for VO from the weaning stage (P21) until puberty onset across all experimental groups.
Blood hormones assays
Blood samples were collected from the heart and centrifuged at 3000 × g for 15 min. The plasma, obtained at 11 A.M., was stored at −80 °C until analyses. Various hormonal levels were determined using commercial ELISA kits from HAKATA, China. Plasma LH levels were assessed with a kit (HZ-030192) featuring a sensitivity of 1 mIU/ml. Plasma FSH levels were measured using a kit (HZ-030195) with a sensitivity of 1 mIU/ml. Plasma estradiol (E2) levels were determined with a kit (HZ-030188) offering a sensitivity of 1 pmol/ml. Plasma insulin levels were quantified with a kit (HZ-030681) having a sensitivity of 1 mIU/L. Leptin levels were assessed using a kit (HZ-030555) with a sensitivity of 1 ng/ml. Plasma GH levels were measured with a kit (HZ-030777) featuring a sensitivity of 1 ng/ml. Plasma IGF-1 levels were determined using a kit (HR-010992) with a sensitivity of 1 ng/ml. All assays were conducted following the manufacturer’s protocols.
Growth hormone treatment
Mice under ADF regimen with a control chow diet were stratified into two groups for recombinant human growth hormone (rhGH) treatment. The rhGH, dissolved in saline, was dosed based on prior studies [30, 31]. Huh et al. used both 1 and 2 IU/kg/day [30], Lama et al. used 1 IU/kg/day [31], and to ensure efficacy, we opted for 2 IU/kg/day. One group received daily subcutaneous injections of 2 IU/kg in the afternoon (3–4 P.M.), while the other group received subcutaneous injections of saline. The dosage was formulated to deliver 10 μL of saline with or without GH per gram of body weight. Additionally, a higher subcutaneous dose of 4 IU/kg/day was explored to evaluate potential increased effectiveness. The rhGH was purchased from GenSci in Changchun, Jilin Province, China.
Statistical analyses
Data are presented as mean ± SEM. Outliers were excluded using the Iterative Grubbs test. To assess Gaussian distribution, normality tests (Kolmogorov–Smirnov test, Shapiro–Wilk test) were conducted. Two populations with normal distributions were compared using unpaired two-tailed t-tests. For datasets with non-Gaussian distributions or significantly different variances (indicated by a significant P value in the F test), unpaired two-tailed Mann–Whitney tests were applied. One-way ANOVA, followed by Tukey’s post hoc multiple-comparison test, was employed for populations with Gaussian distribution. In cases of non-Gaussian distributions or populations with significantly different standard deviations (indicated by a significant P value after the Brown–Forsythe test or Bartlett’s test), Kruskal–Wallis tests were performed, followed by Dunn’s post hoc test for multiple comparisons. Data analyses were carried out using GraphPad Prism Software v.8, and a p-value < 0.05 was considered significant.
Results
18 h ADF reduces body growth and delays puberty onset in normal-weight mice
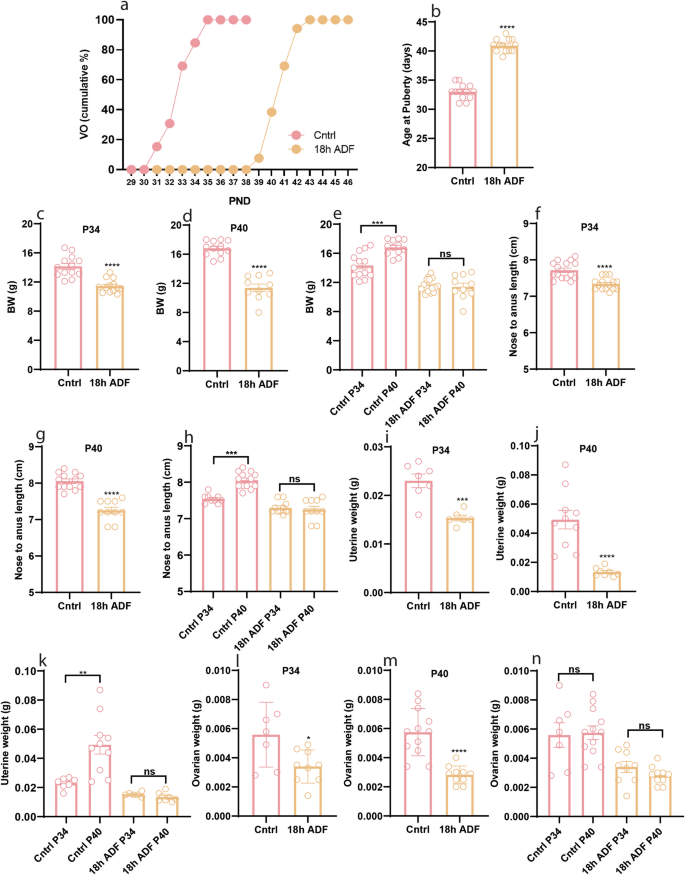
While ADF’s impact on reproduction in female rats is documented [20], it has not been studied whether it affects the onset of puberty in mice. To address this question, we exposed postweaning female mice fed a control chow diet to an 18 h ADF paradigm and examined its effect on puberty onset. Interestingly, ADF delayed puberty onset (Fig. 1a, b, P < 0.0001) and reduced body weight (Fig. 1c, P < 0.0001 & Fig. 1d, P < 0.0001) and body length (Fig. 1f, P < 0.0001, & Fig. 1g, P < 0.0001). Furthermore, ADF-treated mice also showed reduced uterine (Fig. 1i, P = 0.0006, & Fig. 1j, P < 0.0001) and ovarian (Fig. 1l, P = 0.0224 & Fig. 1m, P < 0.0001) weight compared to the Cntrl group. Interestingly, body weight (Fig. 1e, P = 0.0005), body length (Fig. 1h, P = 0.0006), and uterine weight (Fig. 1k, P = 0.0028) increased from P34 toward P40 in Cntrl group but, did not change in mice exposed to ADF. Our results demonstrate that the peripubertal period is very sensitive towards body energy status; therefore, food restriction during this period delays pubertal development.

a Cumulative percentage of VO; b Average age at puberty onset (indicated by VO); c–e Body weight at P34 and P40; f–h Body length at P34 and P40; i–k Uterine weight at P34 and P40; l–n Ovarian weight at P34 and P40. Data are presented as mean ± SEM, and p-values were calculated using the unpaired Student’s t test and the Mann–Whitney test. Each data point corresponds to an individual mouse. PND Postnatal days. The data represent three separate experiments.
18 h ADF reduces reproductive hormone levels in normal-weight mice
To unravel the mechanistic underpinnings of delayed puberty in our animal model, we scrutinized the plasma levels of reproductive hormones LH, FSH, and E2, which are key regulators of female pubertal development [32]. Comparing the ADF group to the Cntrl, plasma LH levels were similar at P34 (Fig. 2a, P = 0.6053). However, the ADF group exhibited elevated plasma LH levels at P40 (Fig. 2b, P = 0.0053) compared to the Cntrl group. Intriguingly, the ADF group demonstrated an increase in plasma LH levels from P34 to P40 (Fig. 2c, P = 0.0296). Conversely, significant reductions were observed in plasma FSH (Fig. 2d, P = 0.0059) and E2 levels (Fig. 2g, P < 0.0001) in the ADF group compared to the Cntrl group at P34, while no substantial differences were noted at P40 (Fig. 2e, P = 0.4479 & Fig. 2h, P = 0.1262). Notably, in the Cntrl group, FSH and E2 levels decreased from P34 to P40 (Fig. 2f, P = 0.0147 & Fig. 2i, P < 0.0001), whereas ADF group maintained comparable levels between P34 and P40 (Fig. 2f, P = 0.2077 & Fig. 2i, P = 0.8665). Our findings suggest that ADF-induced reduction in plasma FSH and E2 levels may contribute to the delayed onset of puberty.
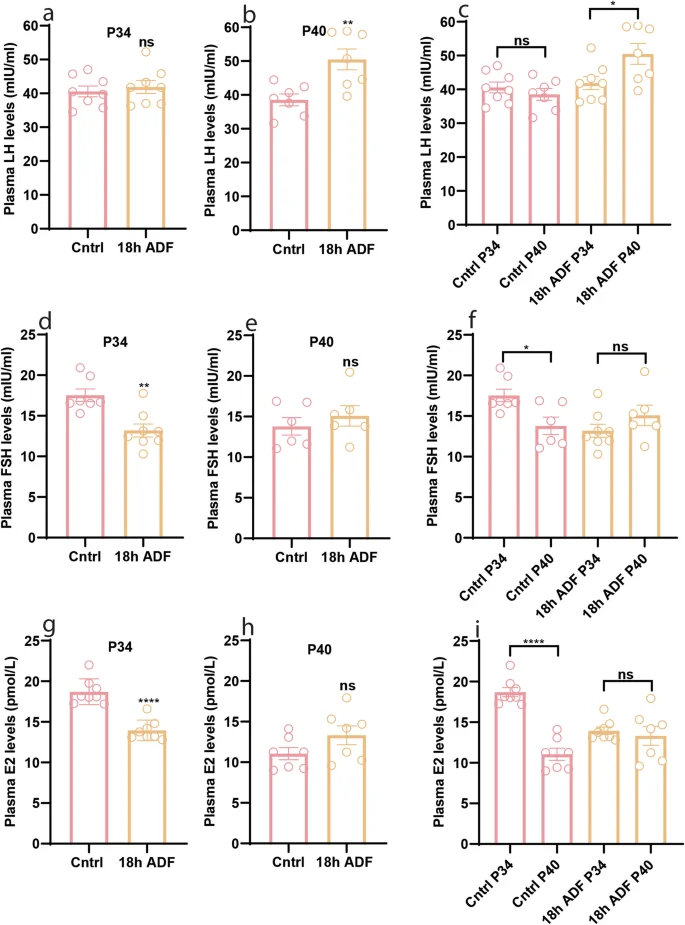
a–c Plasma levels of LH at P34 and P40; d–f Plasma levels of FSH at P34 and P40; g–i Plasma levels of E2 at P34 and P40. Data are presented as mean ± SEM, and p-values were calculated using the unpaired Student’s t test and the Mann–Whitney test. Each data point corresponds to an individual mouse. The data represent two separate experiments.
Effects of 18 h ADF on peripheral hormones regulating body metabolism and growth in normal-weight mice
Leptin and insulin, key peripheral metabolic hormones linked to nutritional status, play roles in regulating reproduction [33,34,35,36]. Investigating the impact of 18 h ADF on these hormones provides insights into the mechanism behind 18 h ADF-induced delayed puberty. Plasma leptin levels showed no significant difference between the Cntrl and ADF groups at P34 (Fig. 3a, P = 0.7546) and P40 (Fig. 3b, P = 0.3824). However, both groups exhibited a reduction in plasma leptin levels from P34 to P40 (Fig. 3c, P < 0.0001 & P = 0.0004). Similarly, no significant difference was observed between the Cntrl and ADF groups in plasma insulin levels at P34 (Fig. 3d, P = 0.6038) and P40 (Fig. 3e, P = 0.2343). Additionally, plasma insulin levels remained unchanged from P34 to P40 within both groups (Fig. 3f, P = 0.8404 & 0.4698). Given that ADF influenced body growth, we explored plasma levels of GH and IGF-1. ADF group showed reduced GH at P34 (Fig. 3g, P = 0.0317) but increased GH at P40 (Fig. 3h, P = 0.0075). Compared to the control group, IGF-1 levels in the ADF group were reduced at P34, although not significantly (Fig. 3j, P = 0.0939). However, by P40, IGF-1 levels were comparable between the groups (Fig. 3k, P = 0.2523). GH levels decreased from P34 to P40 in the Cntrl group but remained consistent between the time points in the ADF group (Fig. 3i, P < 0.0001 & P = 0.8479). Conversely, plasma IGF-1 levels decreased from P34 to P40 in both the Cntrl and ADF groups (Fig. 3l, P = 0.0013 & P < 0.0001). Our results suggest that reduced plasma levels of FSH, E2, and GH/IGF-1 may contribute to delayed pubertal development.
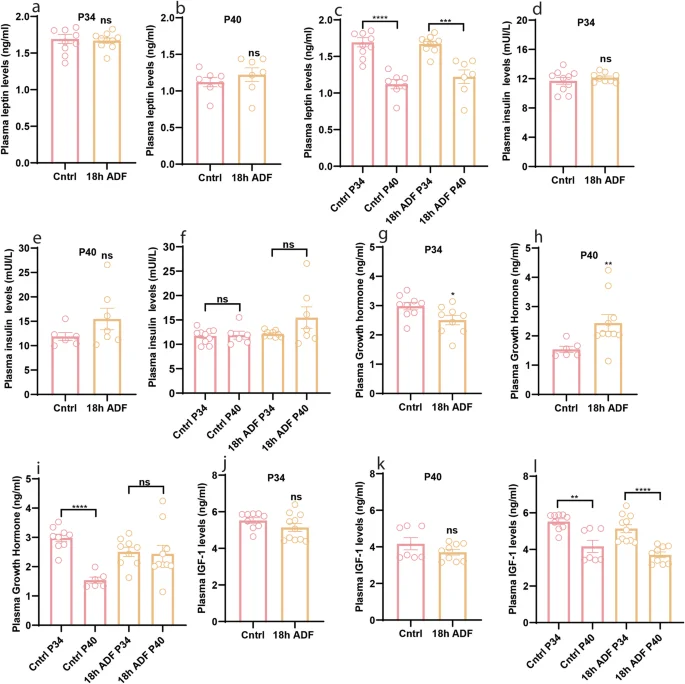
a–c Plasma levels of leptin at P34 and P40; d–f Plasma levels of insulin at P34 and P40; g–i Plasma levels of GH at P34 and P40; j–l Plasma levels of IGF-1 at P34 and P40. Data are presented as mean ± SEM, and p-values were calculated using the unpaired Student’s t test and the Mann–Whitney test. Each data point corresponds to an individual mouse. The data represent two separate experiments.
18 h ADF paradigm prevents HFD-induced obesity but does not prevent precocious puberty
Our previous findings demonstrated that postnatal HFD feeding induces obesity and precocious puberty in female mice [5, 6]. Given the observed effects of 18 h ADF in reducing body weight and length and delaying puberty onset in normal-weight female mice, we hypothesized that 18 h ADF could prevent HFD-induced accelerated body growth and precocious puberty. To test this hypothesis, we exposed HFD-fed mice to 18 h ADF. We found that 18 h ADF did not prevent HFD-induced precocious puberty (Fig. 4a, b, P = 0.2398); however, it did prevent changes in body weight (Fig. 4d, P = 0.0408), and body length (Fig. 4e, P = 0.0477) but not uterine weight (Fig. 4f, P = 0.9550) in HFD-fed mice. Intriguingly, 18 h ADF did not prevent changes in plasma LH levels (Fig. 4h, P = 0.8541), but it did prevent changes in the plasma levels of FSH (Fig. 4i, P = 0.0132) and E2 (Fig. 4j, P = 0.0777). On the other hand, compared to HFD, 18 h ADF did not change plasma levels of GH (Fig. 4k, P = 0.2622) and IGF-1 (Fig. 4l, P = 0.1534). Our results suggest that while 18 h ADF may effectively counteract HFD-induced obesity, it might not be sufficient to prevent precocious puberty.
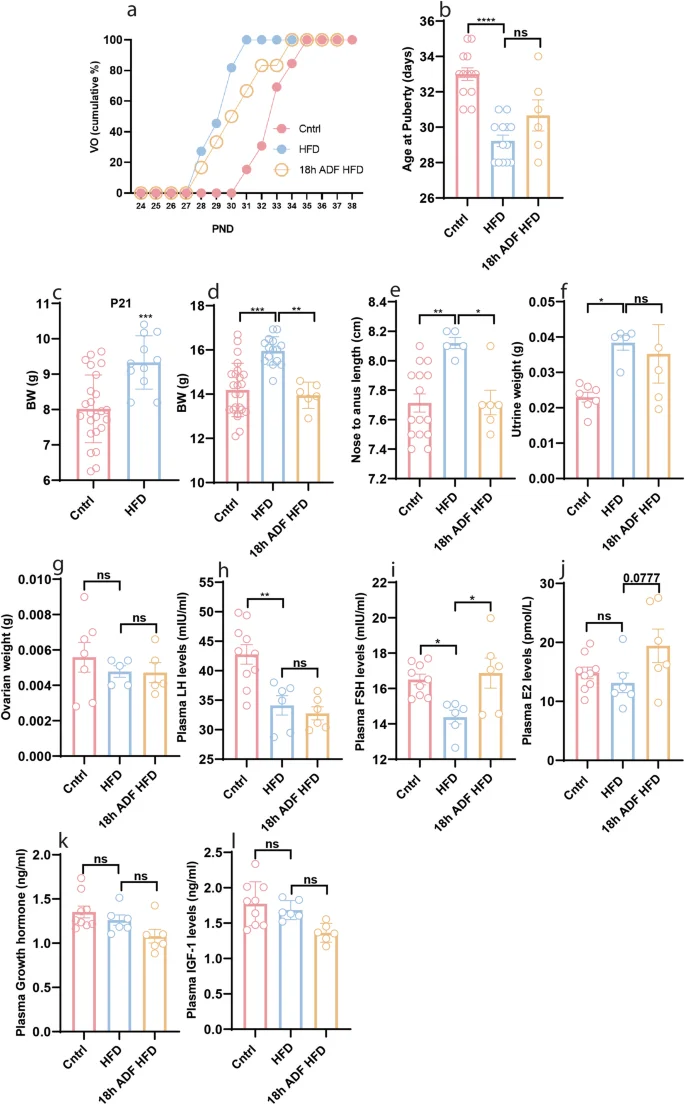
a Cumulative percentage of VO; b Average age at puberty onset; c Body weight at P21; d Body weight at P34; e Body length at P34; f Uterine weight at P34; g Ovarian weight at P34; h Plasma levels of LH at P34; i Plasma levels of FSH at P34; j Plasma levels of E2 at P34; k Plasma levels of GH at P34; l Plasma levels of IGF-1 at P34. Data are presented as mean ± SEM, and p-values were calculated using one-way ANOVA and the Kruskal–Wallis test. Each data point corresponds to an individual mouse. PND Postnatal days. The data represent two separate experiments.
24 h ADF paradigm prevents HFD-induced obesity and precocious puberty
In the preceding data, 18 h ADF prevented changes in E2 and FSH plasma levels but did not prevent precocious puberty in HFD-fed mice. This finding prompted us to explore the potential of a prolonged fasting duration in preventing HFD-induced precocious puberty. Consequently, we exposed female mice fed with HFD to a 24 h ADF paradigm. Intriguingly, the 24 h ADF paradigm successfully prevented HFD-induced precocious puberty (Fig. 5a, b, P < 0.0001) and counteracted changes in body weight (Fig. 5c, P = 0.0005) and length (Fig. 5d, P = < 0.0001). To decipher the underlying mechanism, we assessed reproductive organs and plasma levels of reproductive hormones. The 24 h ADF paradigm prevented HFD-induced increased uterine weight (Fig. 5e, P = 0.0003); however, neither HFD nor 24 h ADF had any effect on ovarian weight (Fig. 5f, P > 0.9999). Furthermore, 24 h ADF had no effects on plasma LH levels (Fig. 5g, P = 0.9927); however, it reinstated HFD-induced alterations in plasma levels of FSH (Fig. 5h, P < 0.0001) and E2 (Fig. 5i, P < 0.0001). We also assessed plasma levels of GH, IGF-1, insulin, and leptin, which are known to play significant roles in body growth, obesity, and reproduction [1, 37, 38]. Interestingly, neither HFD nor 24 h ADF altered plasma levels of GH (Fig. 5j, P = 0.5386 & 0.2181), IGF-1 (Fig. 5k, P = 0.8099 & 0.9045), and insulin (Fig. 5l, P = 0.6981 & >0.9999); however, 24 h ADF prevented HFD-induced elevated levels of leptin (Fig. 5m, P = 0.0020). These data suggest the roles of E2, FSH, and leptin in HFD-induced obesity and precocious puberty, as well as accelerated body growth.
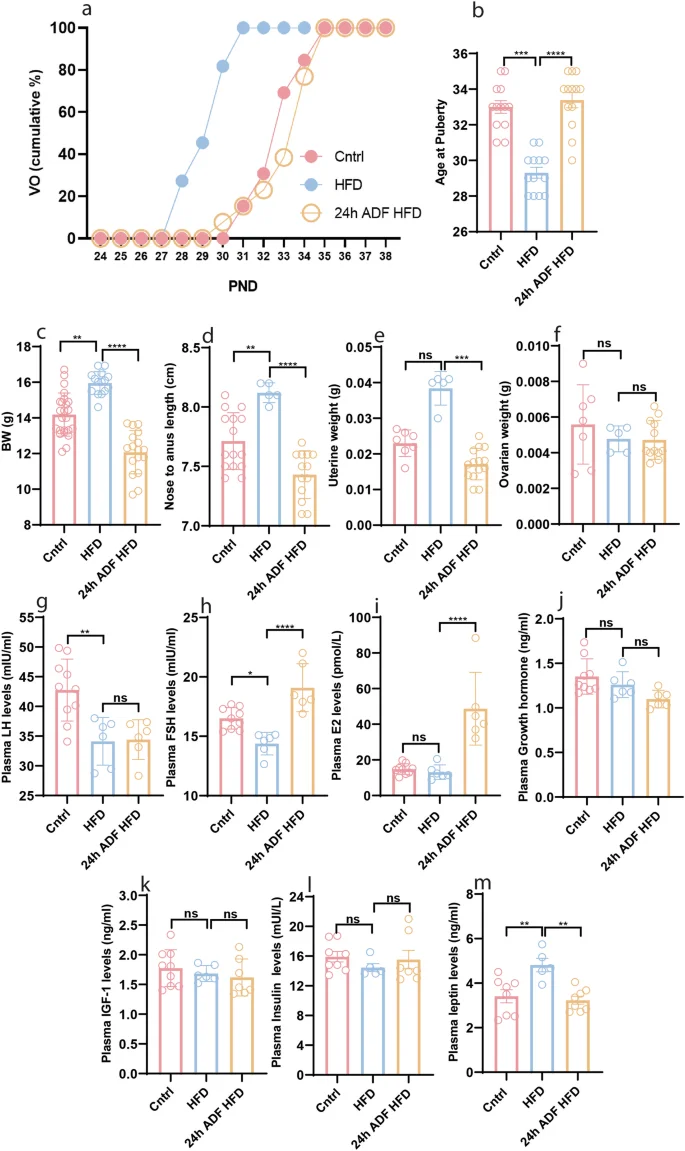
a Cumulative percentage of VO; b Average age at puberty onset; c Body weight at P34; d Body length at P34; e Uterine weight at P34; f Ovarian weight at P34; g Plasma levels of LH at P34; h Plasma levels of FSH at P34; i Plasma levels of E2 at P34; j Plasma levels of GH at P34; k Plasma levels of IGF-1 at P34; l Plasma levels of insulin at P34; m Plasma levels of leptin at P34. Data are presented as mean ± SEM, and p-values were calculated using the one-way ANOVA and the Kruskal–Wallis test. Due to differences in variance, the Kruskal–Wallis test did not indicate a significant difference in uterine weight between the Cntrl and HFD groups, but the Mann–Whitney test did (Supplementary Fig. 2). Each data point corresponds to an individual mouse. PND Postnatal days. The data represent three separate experiments.
To investigate why 24 h ADF effectively prevents HFD-induced precocious puberty while 18 h ADF does not, we compared various parameters related to puberty onset between the two paradigms. Our results showed that, compared to HFD, 24 h ADF significantly reduced uterine weight (Supplementary Fig. 4c, P = 0.0005) and plasma LH levels (Supplementary Fig. 4e, P < 0.0001), and increased plasma levels of FSH (Supplementary Fig. 4f, P = 0.0001) and E2 (Supplementary Fig. 4g, P < 0.0001). In contrast, 18 h ADF did not significantly change these parameters (Supplementary Fig. 4c, P > 0.9999; Fig. 4e, P = 0.9349; Fig. 4g, P = 0.7227), except for a modest increase in plasma FSH levels (Supplementary Fig. 4f, P = 0.0433). Furthermore, 24 h ADF was more potent than 18 h ADF in increasing plasma FSH levels (Supplementary Fig. 4f, P = 0.0825). However, neither HFD (compared to control), nor 18 h or 24 h ADF (compared to HFD) had any significant effects on plasma levels of GH (Supplementary Fig. 4h, P = 0.7288, 0.2622, & 0.9967) or IGF-1 (Supplementary Fig. 4i, P = 0.9041, 0.1534, & 0.9656). These data indicate that the more potent effects of 24 h ADF on uterine weight and plasma levels of LH, FSH, and E2 contribute to the prevention of precocious puberty in HFD feeding mice.
18 h ADF-induced reduced GH levels contribute to reduced body growth but not delayed puberty in normal-weight mice
We observed that neither HFD altered GH/IGF-1 levels, nor did 24 h ADF have any effects on them in HFD-fed mice (Fig. 5j, k). In contrast, HFD increased plasma leptin levels and accelerated pubertal development, and 24 h ADF counteracted all these changes (Fig. 5a, b, m). Given leptin’s well-known role in puberty, our results suggest that, instead of GH/IGF-1, leptin is the principal mediator of HFD-induced precocious puberty. However, 18 h ADF had no effect on plasma leptin levels; rather, it reduced plasma GH/IGF-1 levels and delayed pubertal development in normal weight mice. This implies that, instead of leptin, GH/IGF-1 may contribute to the delayed pubertal development induced by 18 h ADF in normal weight mice. As IGF-1 is the downstream signaling mediator of GH, we injected GH into control female mice exposed to 18 h ADF to investigate the role of the GH/IGF-1 axis in contributing to delayed puberty. GH treatment did not prevent 18 h ADF-induced delayed puberty (Fig. 6a, b, P = 0.7446 & P > 0.9999) and reduced uterine weight (Fig. 6e, P > 0.9999) and ovarian weight (Fig. 6f, P > 0.9999); however, it improved 18 h ADF-induced reduced body weight (Fig. 6c, P = 0.0053) and body length (Fig. 6d, P = 0.0008 & P < 0.0001). Since the 2IU dosage of GH was sufficient to improve body growth but not delayed puberty, we also used the higher dose of 4IU. However, 4IU also improved body growth but did not prevent delayed pubertal onset. Therefore, our results demonstrate that the 18 h ADF-induced reduced levels of GH contribute to reduced body growth; however, reduced levels of sex hormones contribute to delayed puberty.
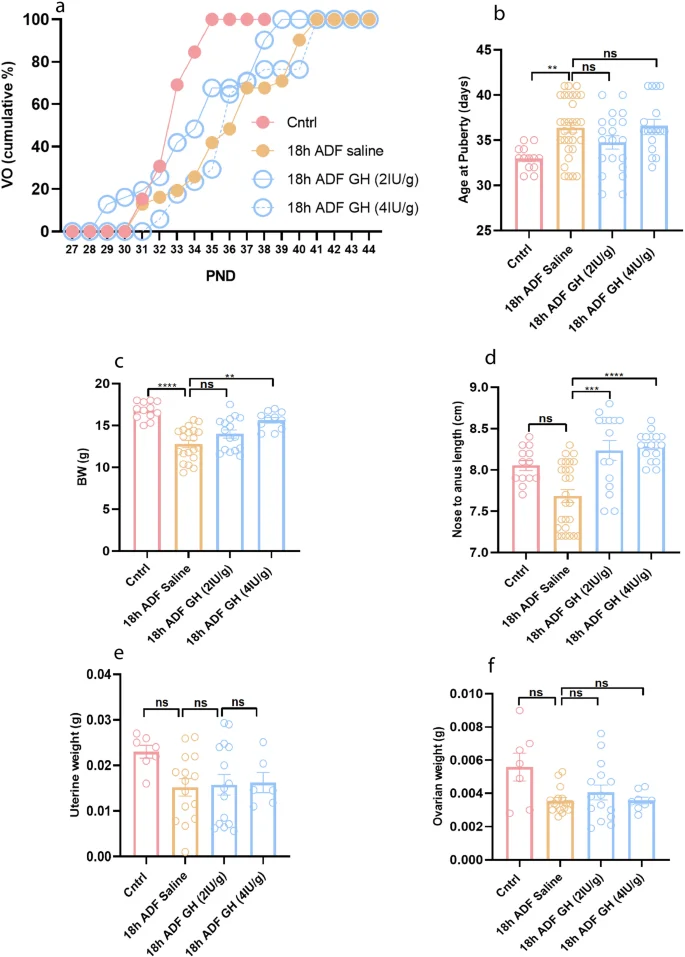
a Cumulative percentage of VO; b Average age at puberty onset; c Body weight at P40; d Body length at P40; e Uterine weight at P40; f Ovarian weight at P40. Data are presented as mean ± SEM, and p-values were calculated using the Kruskal–Wallis test. Due to variance differences, the Kruskal–Wallis test did not indicate significance for nose-to-anus length, uterine weight, and ovarian weight between the Cntrl and HFD groups. However, the Mann–Whitney and unpaired t-tests did show significance (Supplementary Fig. 3). Each data point corresponds to an individual mouse. PND Postnatal days. The data represent three separate experiments.
Discussion
Childhood obesity poses a dual challenge, affecting immediate health in children and imprinting lasting changes that amplify susceptibility to adult diseases [39]. Additionally, childhood obesity is linked to precocious puberty, particularly in girls [40], posing as a risk factor for angina, type 2 diabetes, hypertension [41], other cardiovascular diseases [2], various cancers [42, 43], short adult height [44, 45], psychological maturation, and reduced overall life expectancy [2]. Consequently, the imperative arises to effectively manage childhood obesity and precocious puberty. While medicine-based protocols exist, they carry side effects and may lead to complications. For example, Gonadotropin-releasing hormone agonist treatment, effective in controlling central precocious puberty, has controversial effects on body weight, fat, muscles, bone, insulin signaling, and androgen levels [14,15,16,17,18,19], posing potential risk factors for complications. There is a pressing need for alternative, cost-effective, and health-friendly approaches to address childhood obesity and precocious puberty. Weight loss has been linked to reduced obesity-related health risks, with maximum benefits observed when weight loss occurs before puberty onset [39]. Our study demonstrates that ADF effectively prevents HFD-induced accelerated body growth and precocious puberty in female mice, suggesting it as a safe alternative for managing both obesity and obesity-associated earlier pubertal development.
ADF has demonstrated reproductive inhibition and reduced body weight [20]. However, the impact of ADF on pubertal development in immature normal-weight and diet-induced obese female mice remains unknown. Intriguingly, our data revealed that an 18 h ADF in immature normal-weight female mice led to delayed puberty and reduced ovarian and uterine weight. Consistent with studies in immature rats subjected to undernutrition [46,47,48] and 72 h fasting [49], we observed decreased plasma levels of gonadotropins and E2 in normal-weight female mice exposed to 18 h ADF. Since E2 mediates vaginal canalization through apoptosis [50], the reduced FSH and E2 levels in normal-weight mice exposed to 18 h ADF provide an explanation for the observed delayed puberty. Interestingly, ADF did not alter plasma leptin and insulin levels, metabolic hormones governing metabolism and reproduction [1, 37]. Our findings propose that, rather than metabolic hormones, ADF specifically reduces plasma levels of sex hormones, contributing to delayed pubertal development. As plasma sex hormone levels rise towards puberty and decline afterward [51], the observed reduction in FSH and E2 at P40 in our control mice aligns with normal physiological changes. The increasing trend of FSH and elevated LH levels in the ADF group at P40 may be attributed to pubertal onset. Additionally, in line with undernutrition paradigms [52], we noted reduced body weight and length, and lower plasma levels of GH and IGF-1 in normal-weight mice exposed to ADF. The observed decrease in GH levels from P34 to P40 in control mice aligns with a previous study indicating declining GH levels post-puberty onset [51]. GH regulates metabolism and somatic development, stimulating IGF-1 synthesis, crucial for various functions, including bone growth [38, 53, 54]. Genetic models of GH and GH receptor knockout mice exhibited impaired growth and metabolism [55, 56]. Similarly, subnutrition has been associated with reduced plasma GH levels in rodents [52]. These collective findings suggest that ADF reduces plasma GH levels, contributing to impaired body growth.
In line with our prior research [5, 6], postnatal HFD feeding consistently accelerated body growth, induced precocious puberty, and led to reduced plasma levels of FSH and E2. Sex hormone levels typically rise leading up to puberty and decline afterward [51]. Our earlier study noted upregulated sex hormone levels pre-puberty, followed by unchanged levels at puberty in HFD-fed mice [5]. Collectively, these findings imply that HFD activates an early surge of sex hormones, triggering precocious puberty, with levels returning to baseline once puberty is established. Given that puberty occurs around P29 in HFD-fed and P33 in control mice [5, 6], and sex hormone levels decrease post-puberty onset [51], the observed reduction in sex hormone levels at P34 in HFD-fed mice aligns with this pattern. Notably, our data showed no alterations in plasma levels of GH and IGF-1, indicating that HFD feeding accelerates body growth independently of GH and IGF-1. The 18 h ADF paradigm effectively counteracts body weight and length in HFD-fed mice but did not prevent HFD-induced precocious puberty. Although the reducing trend in uterine weight and sex hormones suggests potential, extending fasting duration may be necessary for preventing HFD-induced precocious puberty.
To counter HFD-induced precocious puberty, we employed the 24 h ADF paradigm, a well-established protocol for managing obesity and related metabolic issues [57,58,59]. Intriguingly, the 24 h ADF paradigm not only successfully prevented HFD-induced accelerated body growth but also precocious puberty. It effectively countered the HFD-induced increase in uterine weight while normalizing levels of FSH and E2. These findings suggest that the 24 h ADF paradigm prevents the premature activation of sex hormones, contributing to the prevention of precocious puberty in HFD-fed mice. Leptin levels are intricately linked to body mass index [60], circulating in accordance with body fat stores [61]. Adequate body fat is pivotal for puberty initiation [62,63,64] with leptin acting as a permissive factor for puberty onset [65]. Our data revealed elevated leptin levels in HFD-fed mice, which were prevented by the 24 h ADF paradigm. Notably, neither HFD nor 24 h ADF affected plasma levels of insulin, GH, and IGF-1. This suggests that HFD induces obesity and elevated leptin, potentially contributing to precocious puberty. Our findings align with studies associating higher body fat and leptin levels with earlier menarche [66]. Leptin, known to influence bone development [67], may also play a role in accelerated growth in HFD-fed mice.
HFD feeding induces elevated plasma leptin levels, alters gonadotropin levels, and triggers obesity and precocious puberty. These effects are successfully prevented by the 24 h ADF paradigm. Neither HFD alone nor HFD with ADF induces changes in GH and IGF-1, indicating that leptin, rather than GH/IGF-1, contributes to HFD-induced accelerated body growth and precocious puberty. In contrast, the 18 h ADF paradigm results in reduced body weight and length, delayed puberty onset, and lowered plasma levels of GH and IGF-1 in mice fed a control chow diet. While GH/IGF-1 are known to mediate nutrition-related effects on linear growth [reviewed in ref. [68]], their role in puberty is less understood. To explore the role of GH in puberty, we administered GH to normal-weight mice exposed to 18 h ADF. GH treatment successfully prevented the 18 h ADF-induced reduced body growth, but it did not prevent the reduction in uterine weight and delayed puberty. Our results are supported by previous studies, where 6 h, 18 h, and 24 h fasting reduced plasma GH levels and growth hormone-releasing hormone expression within the hypothalamus in rodents [69,70,71,72,73]. Taken together, our results suggest that reduced sex hormone levels contribute to delayed puberty and reduced GH levels may contribute to reduced body growth in control mice exposed to 18 h ADF.
Conclusion
In summary, our research indicates that ADF delays puberty in normal-weight mice by influencing sex hormones and growth factors. An HFD induces obesity and precocious puberty, but 24 h ADF prevents these effects. Notably, an 18 h ADF counteracts accelerated body growth caused by HFD but doesn’t prevent precocious puberty. For obese children, starting an 18 h ADF regimen before puberty onset may be a practical preventive measure, considering the prolonged pubertal window in humans. However, this approach also delays puberty and reduces growth in normal-weight mice, prompting caution for parents of such children. HFD-induced obesity triggers metabolic inflammation in the hypothalamus, impacting feeding circuits and contributing to hyperphagia and glucose intolerance. This study does not address whether ADF effectively corrects these effects induced by HFD feeding. However, we plan to investigate this aspect in future studies. While promising for managing diet-induced childhood obesity and precocious puberty, translating ADF to clinical practice requires further research and clinical trials to ensure safety and efficacy in humans.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary files.
References
-
Ullah R, Shen Y, Zhou YD, Fu J. Perinatal metabolic inflammation in the hypothalamus impairs the development of homeostatic feeding circuitry. Metabolism. 2023;147:155677.
Google Scholar
-
Lakshman R, Forouhi NG, Sharp SJ, Luben R, Bingham SA, Khaw KT, et al. Early age at menarche associated with cardiovascular disease and mortality. J Clin Endocrinol Metab. 2009;94:4953–60.
Google Scholar
-
Maione L, Bouvattier C, Kaiser UB. Central precocious puberty: Recent advances in understanding the aetiology and in the clinical approach. Clin Endocrinol. 2021;95:542–55.
-
Liang X, Huang K, Dong G, Chen R, Chen S, Zheng R, et al. Current pubertal development in Chinese children and the impact of overnutrition, lifestyle and perinatal factors. J Clin Endocrinol Metab. 2023;108:2282–9.
-
Ullah R, Raza A, Rauf N, Shen Y, Zhou YD, Fu J. Postnatal Feeding With a Fat Rich Diet Induces Precocious Puberty Independent of Body Weight, Body Fat, and Leptin Levels in Female Mice. Front Endocrinol. 2019;10:758.
-
Ullah R, Su Y, Shen Y, Li C, Xu X, Zhang J, et al. Postnatal feeding with high-fat diet induces obesity and precocious puberty in C57BL/6J mouse pups: a novel model of obesity and puberty. Front Med. 2017;11:266–76.
-
Smith JT, Spencer SJ. Preweaning Over- and Underfeeding Alters Onset of Puberty in the Rat Without Affecting Kisspeptin1. Biol Reprod. 2012;86:1–8.
-
Hong Y, Ullah R, Wang JB, Fu JF. Trends of obesity and overweight among children and adolescents in China. World J Pediatr. 2023;19:1115–26.
Google Scholar
-
Kota AS, Ejaz S. Precocious Puberty. StatPearls. Treasure Island: StatPearls Publishing LLC; 2023.
-
Brown DB, Loomba-Albrecht LA, Bremer AA. Sexual precocity and its treatment. World J Pediatr. 2013;9:103–11.
Google Scholar
-
Dong Y, Qi Y, Jiang H, Mi T, Zhang Y, Peng C, et al. The development and benefits of metformin in various diseases. Front Med. 2023;17:388–431.
Google Scholar
-
Liu Y, Luo X. New practice in semaglutide on type-2 diabetes and obesity: clinical evidence and expectation. Front Med. 2022;16:17–24.
Google Scholar
-
Li X, Li X, Wang G, Xu Y, Wang Y, Hao R, et al. Xiao Ke Qing improves glycometabolism and ameliorates insulin resistance by regulating the PI3K/Akt pathway in KKAy mice. Front Med. 2018;12:688–96.
Google Scholar
-
Głąb E, Wikiera B, Bieniasz J, Barg E. The Influence of GnRH Analog Therapy on Growth in Central Precocious Puberty. Adv Clin Exp Med. 2016;25:27–32.
Google Scholar
-
Arrigo T, De Luca F, Antoniazzi F, Galluzzi F, Segni M, Rosano M, et al. Reduction of baseline body mass index under gonadotropin-suppressive therapy in girls with idiopathic precocious puberty. Eur J Endocrinol. 2004;150:533–7.
Google Scholar
-
Faienza MF, Brunetti G, Acquafredda A, Delvecchio M, Lonero A, Gaeta A, et al. Metabolic Outcomes, Bone Health, and Risk of Polycystic Ovary Syndrome in Girls with Idiopathic Central Precocious Puberty Treated with Gonadotropin-Releasing Hormone Analogues. Horm Res Paediatr. 2017;87:162–9.
Google Scholar
-
Loochi SA, Demol S, Nagelberg N, Lebenthal Y, Phillip M, Yackobovitch-Gavan M. Gonadotropin releasing hormone analogue therapy in girls with idiopathic precocious puberty/early-fast puberty: dynamics in adiposity indices, eating habits and quality of life. J Pediatr Endocrinol Metab. 2021;34:373–83.
Google Scholar
-
Wijarn P, Poomthavorn P, Khlairit P, Pongratanakul S, Chailurkit L, Mahachoklertwattana P. Short-term effects of gonadotropin-releasing hormone analogue treatment on leptin, ghrelin and peptide YY in girls with central precocious puberty. J Pediatr Endocrinol Metab. 2021;34:479–84.
Google Scholar
-
Ramos CO, Latronico AC, Cukier P, Macedo DB, Bessa DS, Cunha-Silva M, et al. Long-Term Outcomes of Patients with Central Precocious Puberty due to Hypothalamic Hamartoma after GnRHa Treatment: Anthropometric, Metabolic, and Reproductive Aspects. Neuroendocrinology. 2018;106:203–10.
Google Scholar
-
Kumar S, Kaur G. Intermittent fasting dietary restriction regimen negatively influences reproduction in young rats: a study of hypothalamo-hypophysial-gonadal axis. PLoS One. 2013;8:e52416.
Google Scholar
-
Welton S, Minty R, O’Driscoll T, Willms H, Poirier D, Madden S, et al. Intermittent fasting and weight loss: Systematic review. Can Fam physician Med de Famille Canadien 2020;66:117–25.
-
Harris L, Hamilton S, Azevedo LB, Olajide J, De Brún C, Waller G, et al. Intermittent fasting interventions for treatment of overweight and obesity in adults: a systematic review and meta-analysis. JBI Database Syst Rev Implement Rep. 2018;16:507–47.
-
Lowe DA, Wu N, Rohdin-Bibby L, Moore AH, Kelly N, Liu YE, et al. Effects of Time-Restricted Eating on Weight Loss and Other Metabolic Parameters in Women and Men With Overweight and Obesity: The TREAT Randomized Clinical Trial. JAMA Intern Med. 2020;180:1491–9.
Google Scholar
-
Crupi AN, Haase J, Brandhorst S, Longo VD. Periodic and Intermittent Fasting in Diabetes and Cardiovascular Disease. Curr Diab Rep. 2020;20:83.
Google Scholar
-
Zhu S, Surampudi P, Rosharavan B, Chondronikola M. Intermittent fasting as a nutrition approach against obesity and metabolic disease. Curr Opin Clin Nutr Metab Care. 2020;23:387–94.
Google Scholar
-
Zhao F, Li R, Xiao S, Diao H, Viveiros MM, Song X, et al. Postweaning exposure to dietary zearalenone, a mycotoxin, promotes premature onset of puberty and disrupts early pregnancy events in female mice. Toxicological Sci Off J Soc Toxicol. 2013;132:431–42.
Google Scholar
-
Safranski T, Lamberso WR, Keisler DH. Correlations among Three Measures of Puberty in Mice and Relationships with Estradiol Concentration and Ovulation1. Biol Reprod. 1993;48:669–73.
Google Scholar
-
Ojeda SR, Wheaton JE, Jameson HE, McCann SM. The onset of puberty in the female rat: changes in plasma prolactin, gonadotropins, luteinizing hormone-releasing hormone (LHRH), and hypothalamic LHRH content. Endocrinology. 1976;98:630–8.
Google Scholar
-
Firlit MG, Schwartz NB. Uncoupling of vaginal opening and the first ovulation–an indication of an alteration in the pituitary-gonadal axis. Biol Reprod. 1977;16:441–4.
Google Scholar
-
Huh K, Nah WH, Xu Y, Park MJ, Gye MC. Effects of Recombinant Human Growth Hormone on the Onset of Puberty, Leydig Cell Differentiation, Spermatogenesis and Hypothalamic KISS1 Expression in Immature Male Rats. World J Mens Health. 2021;39:381–8.
Google Scholar
-
Rol De Lama MA, Pérez-Romero A, Tresguerres JA, Hermanussen M, Ariznavarreta C. Recombinant human growth hormone enhances tibial growth in peripubertal female rats but not in males. Eur J Endocrinol. 2000;142:517–23.
Google Scholar
-
Avendaño MS, Vazquez MJ, Tena-Sempere M. Disentangling puberty: novel neuroendocrine pathways and mechanisms for the control of mammalian puberty. Hum Reprod Update. 2017;23:737–63.
Google Scholar
-
Bouret SG, Draper SJ, Simerly RB. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science. 2004;304:108–10.
Google Scholar
-
Wauters M, Considine RV, Van Gaal LF. Human leptin: from an adipocyte hormone to an endocrine mediator. Eur J Endocrinol. 2000;143:293–311.
Google Scholar
-
Gueorguiev M, Góth ML, Korbonits M. Leptin and puberty: a review. Pituitary. 2001;4:79–86.
Google Scholar
-
Brill DS, Moenter SM. Androgen receptor antagonism and an insulin sensitizer block the advancement of vaginal opening by high-fat diet in mice. Biol Reprod. 2009;81:1093–8.
Google Scholar
-
Roa J, Tena-Sempere M. Connecting metabolism and reproduction: roles of central energy sensors and key molecular mediators. Mol Cell Endocrinol. 2014;397:4–14.
Google Scholar
-
Blum WF, Alherbish A, Alsagheir A, El Awwa A, Kaplan W, Koledova E, et al. The growth hormone-insulin-like growth factor-I axis in the diagnosis and treatment of growth disorders. Endocr Connect. 2018;7:R212–r22.
Google Scholar
-
Marcus C, Danielsson P, Hagman E. Pediatric obesity-Long-term consequences and effect of weight loss. J Intern Med. 2022;292:870–91.
Google Scholar
-
Calcaterra V, Magenes VC, Hruby C, Siccardo F, Mari A, Cordaro E, et al. Links between Childhood Obesity, High-Fat Diet, and Central Precocious Puberty. Children. 2023;10:241.
-
Day FR, Elks CE, Murray A, Ong KK, Perry JR. Puberty timing associated with diabetes, cardiovascular disease and also diverse health outcomes in men and women: the UK Biobank study. Sci Rep. 2015;5:11208.
Google Scholar
-
Bodicoat DH, Schoemaker MJ, Jones ME, McFadden E, Griffin J, Ashworth A, et al. Timing of pubertal stages and breast cancer risk: the Breakthrough Generations Study. Breast cancer Res. 2014;16:R18.
Google Scholar
-
Forman D, Pike MC, Davey G, Dawson S, Baker K, Chilvers CED, et al. Aetiology of testicular cancer: association with congenital abnormalities, age at puberty, infertility, and exercise. BMJ. 1994;308:1393–9.
-
Klein KO, Barnes KM, Jones JV, Feuillan PP, Cutler GB Jr. Increased Final Height in Precocious Puberty after Long-Term Treatment with LHRH Agonists: The National Institutes of Health Experience. J Clin Endocrinol Metab. 2001;86:4711–6.
Google Scholar
-
Knific T, Lazarevič M, Žibert J, Obolnar N, Aleksovska N, Šuput Omladič J, et al. Final adult height in children with central precocious puberty – a retrospective study. Front Endocrinol (Lausanne). 2022;13:1008474.
Google Scholar
-
Navarro VM, Ruiz-Pino F, Sánchez-Garrido MA, García-Galiano D, Hobbs SJ, Manfredi-Lozano M, et al. Role of neurokinin B in the control of female puberty and its modulation by metabolic status. J Neurosci. 2012;32:2388–97.
Google Scholar
-
Cui LL, Li P, Zhu ZY. Impact of changes in postnatal nutrition on puberty onset and the expression of hypothalamic GnRH and ghrelin. Eur Rev Med Pharm Sci. 2014;18:703–9.
-
Roa J, Garcia-Galiano D, Varela L, Sánchez-Garrido MA, Pineda R, Castellano JM, et al. The Mammalian Target of Rapamycin as Novel Central Regulator of Puberty Onset via Modulation of Hypothalamic Kiss1 System. Endocrinology 2009;150:5016–26.
Google Scholar
-
Luo Q, Li W, Li M, Zhang X, Zhang H. Leptin/leptinR-kisspeptin/kiss1r-GnRH pathway reacting to regulate puberty onset during negative energy balance. Life Sci. 2016;153:207–12.
Google Scholar
-
Ito T, Bai T, Tanaka T, Yoshida K, Ueyama T, Miyajima M, et al. Estrogen-dependent proteolytic cleavage of semaphorin 4D and plexin-B1 enhances semaphorin 4D-induced apoptosis during postnatal vaginal remodeling in pubescent mice. PloS one. 2014;9:e97909.
Google Scholar
-
Haldar A, Prakash BS. Peripheral Patterns of Growth Hormone, Luteinizing Hormone, and Progesterone Before, at, and after Puberty in Buffalo Heifer. Endocr Res. 2005;31:295–306.
Google Scholar
-
Nijjar JK, Stafford D. Undernutrition and growth in the developing world. Curr Opin Endocrinol Diabetes Obes. 2019;26:32–8.
Google Scholar
-
Dehkhoda F, Lee CMM, Medina J, Brooks AJ. The Growth Hormone Receptor: Mechanism of Receptor Activation, Cell Signaling, and Physiological Aspects. Front Endocrinol. 2018;9:35.
-
Olarescu NC, Gunawardane K, Hansen TK, Møller N, Jørgensen JOL. Normal Physiology of Growth Hormone in Adults. In: Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, et al., editors. Endotext. South Dartmouth (MA): MDText.com, Inc.; 2000.
-
List EO, Berryman DE, Buchman M, Jensen EA, Funk K, Duran-Ortiz S, et al. GH Knockout Mice Have Increased Subcutaneous Adipose Tissue With Decreased Fibrosis and Enhanced Insulin Sensitivity. Endocrinology. 2019;160:1743–56.
Google Scholar
-
Zhou Y, Xu BC, Maheshwari HG, He L, Reed M, Lozykowski M, et al. A mammalian model for Laron syndrome produced by targeted disruption of the mouse growth hormone receptor/binding protein gene (the Laron mouse). Proc Natl Acad Sci USA 1997;94:13215–20.
Google Scholar
-
Zhang W, Wang J, Wang L, Shi R, Chu C, Shi Z, et al. Alternate-day fasting prevents non-alcoholic fatty liver disease and working memory impairment in diet-induced obese mice. J Nutritional Biochem. 2022;110:109146.
Google Scholar
-
Yang W, Cao M, Mao X, Wei X, Li X, Chen G, et al. Alternate-day fasting protects the livers of mice against high-fat diet–induced inflammation associated with the suppression of Toll-like receptor 4/nuclear factor κB signaling. Nutr Res. 2016;36:586–93.
Google Scholar
-
Henderson CG, Turner DL, Swoap SJ. Health Effects of Alternate Day Fasting Versus Pair-Fed Caloric Restriction in Diet-Induced Obese C57Bl/6J Male Mice. Front Physiol. 2021;12:641532.
-
Yoo JW, Song CW, Lim HH. Leptin and adiponectin levels in girls with central precocious puberty before and during GnRH agonist treatment. Ann Pediatr Endocrinol Metab. 2016;21:199–205.
Google Scholar
-
Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, et al. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med. 1995;1:1155–61.
Google Scholar
-
Baker ER. Body weight and the initiation of puberty. Clin Obstet Gynecol. 1985;28:573–9.
Google Scholar
-
Adami F, Benedet J, Takahashi LAR, da Silva Lopes A, da Silva Paiva L, de Vasconcelos. FdAG. Association between pubertal development stages and body adiposity in children and adolescents. Health Qual Life Outcomes. 2020;18:93.
Google Scholar
-
Huang L, Hou JW, Fan HY, Tsai MC, Yang C, Hsu JB, et al. Critical body fat percentage required for puberty onset: the Taiwan Pubertal Longitudinal Study. J Endocrinological Investig. 2023;46:1177–85.
Google Scholar
-
Elias CF. Leptin action in pubertal development: recent advances and unanswered questions. Trends Endocrinol Metab. 2012;23:9–15.
Google Scholar
-
Shalitin S, Phillip M. Role of obesity and leptin in the pubertal process and pubertal growth–a review. Int J Obes Relat Metab Disord. 2003;27:869–74.
Google Scholar
-
Maor G, Rochwerger M, Segev Y, Phillip M. Leptin acts as a growth factor on the chondrocytes of skeletal growth centers. J Bone Min Res. 2002;17:1034–43.
Google Scholar
-
Inzaghi E, Pampanini V, Deodati A, Cianfarani S. The Effects of Nutrition on Linear Growth. Nutrients. 2022;14:1752.
-
Steyn FJ, Leong JW, Huang L, Tan HY, Xie TY, Nelson C, et al. GH Does Not Modulate the Early Fasting-Induced Release of Free Fatty Acids in Mice. Endocrinology. 2012;153:273–82.
Google Scholar
-
Steyn FJ, Huang L, Ngo ST, Leong JW, Tan HY, Xie TY, et al. Development of a Method for the Determination of Pulsatile Growth Hormone Secretion in Mice. Endocrinology. 2011;152:3165–71.
Google Scholar
-
Huang L, Tan HY, Fogarty MJ, Andrews ZB, Veldhuis JD, Herzog H, et al. Actions of NPY, and its Y1 and Y2 receptors on pulsatile growth hormone secretion during the fed and fasted state. J Neurosci. 2014;34:16309–19.
Google Scholar
-
Bruno JF, Olchovsky D, White JD, Leidy JW, Song J, Berelowitz M. Influence of food deprivation in the rat on hypothalamic expression of growth hormone-releasing factor and somatostatin. Endocrinology. 1990;127:2111–6.
Google Scholar
-
Tannenbaum GS, Rorstad O, Brazeau P. Effects of prolonged food deprivation on the ultradian growth hormone rhythm and immunoreactive somatostatin tissue levels in the rat. Endocrinology. 1979;104:1733–8.
Google Scholar
Acknowledgements
The author expresses sincere gratitude to the animal facility for their care and support during the experiments. The authors express their gratitude for the financial support provided by the National Key R&D Program of China (No. 2021YFC2701900); Key R&D Program of Zhejiang (No.2023C03047); and National Natural Science Foundation of China (No. 82370863, No. 82350410491).
Author information
Authors and Affiliations
Contributions
Rahim Ullah designed and conducted experiments and wrote the manuscript under the supervision of Yi Shen, Yu-Dong Zhou, and Junfen Fu, actively contributing throughout the entire process. Chuqing Xue, Senjie Wang, Zhewen Qin, Naveed Rauf, and Shumin Zhan assisted during the breeding of mice and in performing experiments. Naveed Rauf and Naimat Ullah Khan contributed the intellectual editing of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study adhered to the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and received approval from the Animal Advisory Committee at Zhejiang University (ethics code: ZJU20210313).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Materilas
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
Reprints and permissions
About this article
Cite this article
Ullah, R., Xue, C., Wang, S. et al. Alternate-day fasting delays pubertal development in normal-weight mice but prevents high-fat diet-induced obesity and precocious puberty.
Nutr. Diabetes 14, 82 (2024). https://doi.org/10.1038/s41387-024-00335-w
-
Received: 21 January 2024
-
Revised: 20 August 2024
-
Accepted: 04 September 2024
-
Published: 04 October 2024
-
DOI: https://doi.org/10.1038/s41387-024-00335-w