
Artificial <span class="glossaryLink" aria-describedby="tt" data-cmtooltip="
” data-gt-translate-attributes=”[{“attribute”:”data-cmtooltip”, “format”:”html”}]” tabindex=”0″ role=”link”>photosynthesis holds the key to cleaner energy and carbon capture, but replicating nature’s process is no easy feat.
A breakthrough from JMU Würzburg researchers has brought science one step closer by creating a stacked dye system that efficiently moves charge carriers using light—just like in plant cells.
Harnessing Sunlight: The Magic of Photosynthesis
Photosynthesis is the process plants use to convert sunlight, carbon dioxide, and water into energy-rich sugars and oxygen. This remarkable system fuels plant growth and releases the oxygen we breathe.
If scientists could replicate photosynthesis, the benefits would be immense. Solar energy could be harnessed to remove carbon dioxide from the air and transform it into valuable compounds like carbohydrates. Additionally, since photosynthesis naturally splits water into oxygen and hydrogen, artificial versions could provide a new way to produce clean hydrogen fuel.
Photosynthesis: A Complex Process With Many Participants
Given its potential, researchers around the world are working to develop artificial photosynthesis. However, replicating nature’s method is a major challenge. The process involves multiple intricate steps within plant cells, relying on a network of pigments, proteins, and other molecules. Despite these complexities, scientific progress in this field continues to accelerate.
One of the leading experts in artificial photosynthesis is Professor Frank Würthner from Julius-Maximilians-Universität (JMU) Würzburg in Germany. His team has successfully mimicked one of the early steps of natural photosynthesis using an advanced arrangement of artificial dye molecules. This breakthrough has provided new insights into how energy is transferred and stored in the process.
This achievement, developed in collaboration with Professor Dongho Kim’s research team at <span class="glossaryLink" aria-describedby="tt" data-cmtooltip="
” data-gt-translate-attributes=”[{“attribute”:”data-cmtooltip”, “format”:”html”}]” tabindex=”0″ role=”link”>Yonsei University in South Korea, was published today (March 14) in Nature Chemistry.
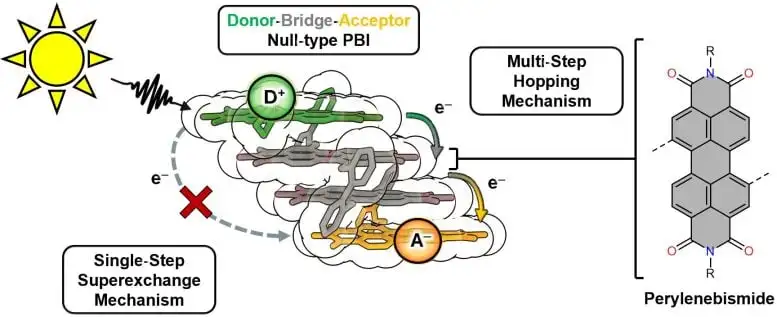
Fast and Efficient Energy Transport in a Stacking System
The researchers have succeeded in synthesizing a stack of dyes that is very similar to the photosynthetic apparatus in plant cells – it absorbs light energy at one end, uses it to separate charge carriers, and transfers them step by step to the other end via a transport of electrons. The structure consists of four stacked dye molecules from the perylene bisimide class.
“We can specifically trigger the charge transport in this structure with light and have analyzed it in detail. It is efficient and fast. This is an important step towards the development of artificial photosynthesis,” says JMU PhD student Leander Ernst, who synthesized the stacked structure.
Supramolecular Wires as the Goal of the Research Work
Next, the JMU research team wants to expand the nanosystem of stacked dye molecules from four to more components – with the aim of ultimately creating a kind of supramolecular wire that absorbs light energy and transports it quickly and efficiently over longer distances. This would be a further step towards novel photofunctional materials that can be used for artificial photosynthesis.
Reference: “Photoinduced stepwise charge hopping in π-stacked perylene bisimide donor-bridge-acceptor arrays” 14 March 2025, Nature Chemistry.
DOI: 10.1038/s41557-025-01770-7
