Abstract
To explore the inflammation-nutrition indices and related clinical factors affecting early recurrence in patients with stage IB LUAD. A retrospective analysis was conducted on clinical and pathological data of patients diagnosed with stage IB LUAD who underwent radical surgery in our hospital from January 2016 to January 2021. Using R software, patients were randomly divided into training (n = 140) and validation (n = 59) cohorts in a 7:3 ratio. Univariate and multivariate Cox regression analyses were performed to identify risk factors for RFS and construct a predictive model. The performance of the model was evaluated using the area under the receiver operating characteristic curve (AUC), concordance index (C-index), and calibration curve. Clinical utility of the model was assessed using decision curve analysis (DCA). Multivariate Cox regression analysis revealed that vascular invasion, visceral pleural invasion, predominant pattern, preoperative NLR > 2.33, preoperative PLR > 127.62, and preoperative PNI ≤ 48.3 were independent risk factors for RFS. The C-index of the nomogram model constructed based on these independent risk factors was 0.825 (95% CI: 0.762–0.881) in the training cohort and 0.772 (95% CI: 0.667–0.876) in the validation cohort. The ROC curves showed AUCs of 0.902, 0.881, and 0.877 for 1-year, 2-year, and 3-year RFS in the training cohort and AUCs of 0.782, 0.825, and 0.732 in the validation cohort respectively. Calibration curve and decision curve analysis indicated good clinical value of the model. The nomogram model based on inflammation-nutrition indices has predictive value for early recurrence in patients with stage IB LUAD.
Introduction
Lung cancer currently ranks as the most common malignancy worldwide in terms of both incidence and mortality, with approximately 1.8 million deaths attributed to it annually1. Non-small cell lung cancer (NSCLC) constitutes the majority of lung cancer cases and includes adenocarcinoma and squamous cell carcinoma, among others, with lung adenocarcinoma (LUAD) being the most prevalent histological subtype2. The preferred treatment for early-stage NSCLC remains anatomical lung resection combined with lymph node dissection, however, up to 30–75% of patients experience local recurrence or distant metastasis following surgery, with stage IB lung cancer accounting for the vast majority3,4. According to the eighth edition of the AJCC staging system, stage IB lung cancer is characterized by a primary lesion measuring 3–4 cm without lymph node or distant metastasis, with a 5-year overall survival (OS) rate of approximately 60%, indicating a significant risk of postoperative recurrence5. Although many studies have identified histological subtypes, spread through air spaces (STAS), vascular invasion and wedge resection as high-risk factors for early recurrence in lung cancer, there is still no model that can accurately predict the early recurrence of stage IB lung cancer, so whether adjuvant chemotherapy is needed after surgery is still a headache for clinicians6,7,8. Recent research suggests cancer prognosis are influenced not only by tumor-related factors but also by patient-related factors, such as immune function, nutritional status and inflammatory responses, which are closely associated with tumor development. A large number of studies have shown that neutrophil-to-lymphocyte ratio (NLR), lymphocyte-to-monocyte ratio (LMR), and prognostic nutritional index (PNI) are associated with the prognosis of various cancers9,10,11,12.
Currently, there is limited literature on the role of inflammation-nutrition indices in the prognosis of stage IB LUAD, so it has great reaserch value. This study aims to analyze the relationship between several preoperative inflammation-nutrition indices and early recurrence in stage IB LUAD, and to construct and validate relevant nomograms, providing valuable insights for clinicians in assessing early recurrence in stage IB LUAD.
Materials and methods
Patient section
A retrospective collection of data was conducted on patients who underwent radical surgery for lung cancer at our hospital from January 2016 to January 2021. Inclusion criteria were as follows: (1) Postoperative pathological diagnosis of primary stage IB LUAD; (2) Negative bronchial margins; (3) Absence of distant metastasis. Exclusion criteria were: (1) Pathological types including mucinous adenocarcinoma, in situ adenocarcinoma, or minimally invasive adenocarcinoma; (2) Previous anti-inflammatory treatment before surgery; (3) History of other malignant tumors; (4) Patients with hematological or autoimmune diseases; (5) Incomplete clinical data. A total of 199 patients were ultimately included in this study and randomly divided into training and validation cohorts in a 7:3 ratio. This study was approved by the Ethics Committee of Li Huili Hospital, Ningbo University (Approval No: KY2019PJ058). Individual informed consent for this retrospective analysis was waived by the Ethics Committee of Li Huili Hospital. All experiments were performed in accordance with relevant guidelines and regulations.
Data collection
Clinical data of 199 patients with stage IB lung adenocarcinoma were collected, including gender, age, smoking history, tumor location, maximum tumor diameter, vascular invasion, nerve invasion, pleural visceral invasion (VPI), predominant pattern, spread through air spaces (STAS), surgical approach, postoperative adjuvant chemotherapy, preoperative neutrophil count, preoperative lymphocyte count, preoperative monocyte count, preoperative platelet count, and preoperative albumin level, the laboratory indicators were collected within one week before the surgery. The definitions of NLR, LMR, PLR and PNI are calculated as follows: NLR = neutrophil count/lymphocyte count; LMR = lymphocyte count/monocyte count; PLR = platelet count/lymphocyte count; and PNI = serum albumin level + 5×lymphocyte count.
Postoperative follow-up
All patients were regularly followed up postoperatively through telephone interviews and outpatient visits to assess their recurrence status and calculate the recurrence-free survival (RFS). RFS refers to the duration from the date of surgery to the first tumor recurrence or the last follow-up date. Postoperative recurrence was primarily diagnosed through chest CT, abdominal CT, cranial MRI, whole-body PET-CT scans, and pathological examinations. Patients were followed up every 6 months within the first 3 years postoperatively and annually thereafter. The last follow-up was conducted in February 2024.
Statistical analysis
Data analysis was performed using SPSS 28.0, R 4.2.0, and GraphPad Prism 10.1.2. ROC curves were generated for NLR, LMR, PLR, and PNI to determine cutoff values. Kaplan-Meier curves were constructed to illustrate survival, and log-rank tests were used for comparisons. Univariate Cox regression analysis was initially conducted to screen for variables influencing RFS, calculating hazard ratios (HR) and 95% confidence intervals (CI). Variables with P < 0.1 in the univariate analysis were further analyzed using multivariate Cox regression to identify independent prognostic factors, upon which a nomogram model for RFS was constructed. The nomogram’s prognostic predictive ability was evaluated and validated using the concordance index (C-index), calibration curve, and decision curve analysis (DCA). Differences were considered statistically significant at P < 0.05.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Results
Clinical and pathological characteristics
The clinical and pathological characteristics of the 199 patients with stage IB LUAD are summarized in Table 1. Among them, there were 94 females (47.2%) and 105 males (52.8%), with a median age of 65 years (range: 58–70 years), and 78 patients had a history of smoking (39.2%). Tumors were located in the upper lobe in 121 cases (60.8%) and in the middle/lower lobes in 78 cases (39.2%), with a median maximum tumor diameter of 3.0 cm (range: 2.2–3.5 cm). Postoperative pathological revealed 47 cases with vascular invasion (23.6%), 11 cases with nerve invasion (5.5%), 101 cases with pleural visceral invasion (50.8%), and 7 cases with STAS (3.5%). Predominant patterns were as follows: lepidic, 47 (23.6%); acinar/papillary, 99 (49.7%); and micropapillary/solid, 53 (26.7%). Among them, 171 patients (85.9%) underwent lobectomy, 28 patients (14.1%) underwent segmental or wedge resection. And 105 patients (52.8%) received postoperative adjuvant chemotherapy. The median preoperative NLR was 1.90 (range: 1.47–2.53), the median LMR was 3.75 (range: 3.00-4.83), the median PLR was 126.0 (range: 100.5–156.0), and the median PNI was 50.0 (range: 47.5–53.1). Among them, there were 140 patients in the training cohort and 59 patients in the validation cohort, with no statistically significant differences in baseline characteristics between the two cohorts (P > 0.05). The median follow-up time was 65.4 months. At the end of the follow-up period, 37 patients had died (18.6%), and 69 patients experienced recurrence (34.7%). The cumulative RFS rates at 1 year, 2 years, and 3 years were 93.47%, 85.93%, and 77.39%, respectively.
Determination of cutoff values for inflammation-nutrition indices and prognostic analysis
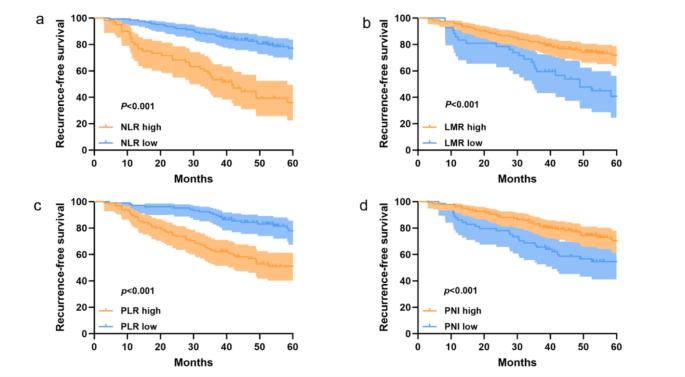
The optimal cutoff values for each inflammation-nutrition indices for recurrence-free survival (RFS) were determined using ROC curves, with NLR, LMR, PLR, and PNI identified as 2.33, 2.71, 127.62, and 48.3 correspoding to the maximum Youden index. Consequently, patients are divided into two groups for further analysis based on the optimal cutoff values. As follows: a low-NLR group (≤ 2.33, n = 137) or a high-NLR group (>2.33, n = 62); a low-LMR group (≤ 2.71, n = 28)or a high-LMR group (>2.71, n = 171); a low-PLR group (≤ 127.62, n = 101)or a high-PLR group (>127.61, n = 98); a low-PNI group (≤ 48.3, n = 70)or a high-PNI group (>48.3, n = 129); Survival analysis was performed using the Kaplan-Meier method for the high and low groups of each inflammation-nutrition indices (Fig. 1), showing statistically significant differences in RFS among all four groups (P < 0.001).

Kaplan-Meier survival curves for RFS, stratified by (a) NLR, (b) LMR, (c) PLR and (d) PNI in LUAD patients with stage IB.
Univariate and multivariate Cox analysis
Univariate Cox regression analysis of the 140 patients in the training cohort revealed that vascular invasion (P < 0.001), nerve invasion (P = 0.048), pleural visceral invasion (P < 0.001), predominant pattern (acinar/papillary, P = 0.003; micropapillary/solid, P = 0.002), preoperative NLR > 2.33 (P < 0.001), preoperative LMR ≤ 2.71 (P 127.62 (P < 0.001), and preoperative PNI ≤ 48.3 (P < 0.001) were associated with poor RFS. However, factors such as age, gender, smoking history, tumor size, tumor location, STAS, surgical approach, and postoperative adjuvant chemotherapy showed no significant relationship with RFS (Table 2). Variables with P < 0.1 in the univariate analysis were included in the multivariate Cox regression analysis (Table 2), which indicated that vascular invasion (P = 0.001), pleural visceral invasion (P = 0.048), predominant pattern (acinar/papillary, P = 0.043; micropapillary/solid, P = 0.044), preoperative NLR > 2.33 (P = 0.001), preoperative PLR > 127.62 (P = 0.001), and preoperative PNI ≤ 48.3 (P = 0.003) were independent risk factors for RFS.
Establishment and validation of the predictive model
A nomogram model was constructed based on the six independent risk factors associated with RFS identified through multivariate Cox regression analysis (Fig. 2). The nomogram exhibited good accuracy in predicting RFS rates, with a C-index of 0.825 (95% CI: 0.762–0.881) in the training cohort and 0.772 (95% CI: 0.667–0.876) in the internal validation cohort. The ROC curve provides an intuitive graphical representation of the model’s performance by demonstrating the trade-off between the true positive rate (TPR) and the false positive rate (FPR). The AUC value is the area below the ROC curve, and the higher the AUC value, the better the prediction of the model. Analysis of the ROC curves for 1-year, 2-year, and 3-year RFS rates (Fig. 3) revealed AUCs of 0.902, 0.881, and 0.877 in the training cohort and 0.782, 0.825, and 0.732 in the internal validation cohort, respectively. The AUC value of the model is high in both the training set and the validation set indicating that the model has a good discrimination for the early recurrence of stage IB lung adenocarcinoma. Then, we plotted the calibration curve. Calibration curve is a visual tool to assess the agreement between predictions and observations in different percentiles of the predicted values. Model validation was further conducted using 1000 bootstrap resamples, and calibration curves were plotted, demonstrating that the predicted 1-year, 2-year, and 3-year RFS rates in both the training and internal validation cohorts were well aligned with the ideal 45-degree line, indicating that the predicted probability of the model is basically consistent with the actual probability (Fig. 4). Additionally, decision curve analysis (DCA) curves were utilized to evaluate the clinical utility of the predictive model by quantifying the net benefit at different threshold probabilities. The DCA curves for predicting 1-year, 2-year, and 3-year RFS rates in both the training and internal validation cohorts were situated in the upper-right quadrant (Fig. 5), suggesting that the nomogram based on inflammation-nutrition indices holds good clinical value for predicting early recurrence in patients with stage IB LUAD.
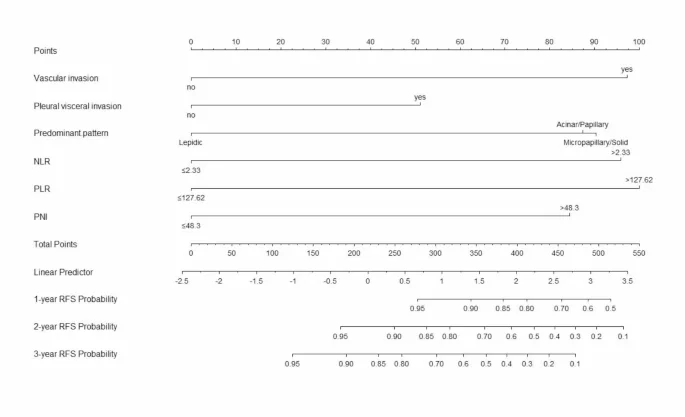
Inflammation-nutritional marker-based nomogram for predicting early recurrence of LUAD patients with stage IB.
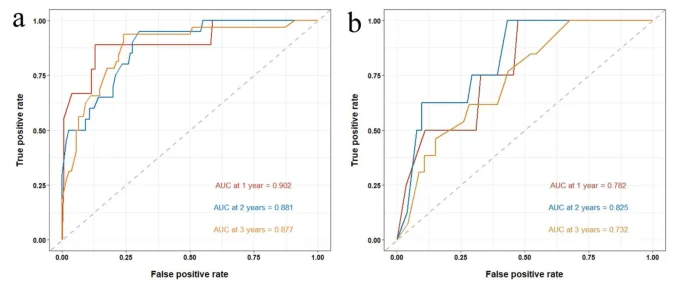
The receiver operating characteristic (ROC) curves for predicting RFS at 1-, 2- and 3-year obtained by using established nomogram in training and internal validation cohort. (a) AUC curves for 1-, 2- and 3-year RFS in training cohort. (b) AUC curves for 1-, 2- and 3-year RFS in internal validation cohort.
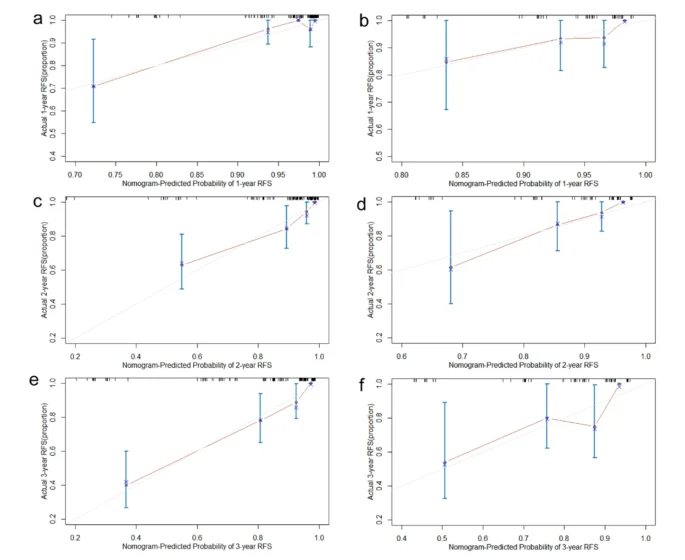
The calibration curve of the nomogram 1-year RFS in training and internal validation cohort. (a and b) The calibration curve of the nomogram 2-year RFS in training and internal validation cohort. (c and d) The calibration curve of the nomogram 1-year RFS in training and internal validation cohort (e and f).
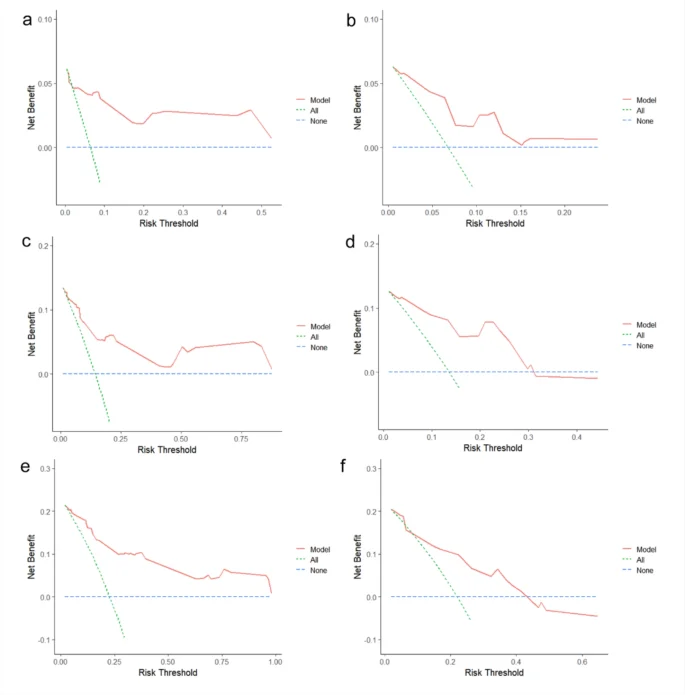
Decision curves for RFS at 1-, 2- and 3-year obtanined by using established nomogram in traning and internal validation cohort. DCA curves for 1-year RFS in training and internal validation cohort. (a and b) DCA curves for 2-year RFS in training and internal validation cohort. (c and d) DCA curves for 3-year RFS in training and internal validation cohort (e and f).
Discussion
Surgical resection remains the preferred treatment for early-stage NSCLC, but outcomes vary significantly, with better prognosis observed in stage IA patients, while the five-year survival rate for stage IB patients is only around 60–80%, indicating a substantial risk of local recurrence and distant metastasis postoperatively. However, there are currently no definitive indicators to accurately predict early recurrence in these patients, highlighting the critical clinical significance of identifying indices for predicting postoperative early recurrence in patients with stage IB LUAD.
In this study, we identified vascular invasion, pleural visceral invasion, predominant pattern, high preoperative NLR, high preoperative PLR, and low preoperative PNI as independent risk factors influencing early recurrence in patients with stage IB LUAD. Consequently, we developed a nomogram based on inflammation-nutrition indices to predict RFS in these patients postoperatively. The nomogram demonstrated precise discriminative and calibration abilities, with AUCs of 0.902, 0.881, and 0.877 for 1-year, 2-year, and 3-year RFS rates in the training cohort, respectively, and AUCs of 0.782, 0.825, and 0.732 for the same time points in the validation cohort. Therefore, this model has some guiding significance for the postoperative treatment of stage IB lung adenocarcinoma patients.
Vascular invasion refers to tumor cell invasion into blood vessels or lymphatic vessels, and several scholars have reported vascular invasion as an independent prognostic factor for lung cancer patients13,14. In a large-scale retrospective study, Wang et al. analyzed clinical data from 2633 stage I NSCLC patients and found that among the 222 patients with vascular invasion, approximately 49.1% were stage IB. Patients with vascular invasion had significantly lower 5-year OS and RFS rates compared to those without vascular invasion, suggesting a significant association between prognosis and vascular invasion in stage IB patients15. The eighth edition of the UICC/AJCC lung cancer TNM staging classifies visceral pleural invasion (VPI) as T2, thereby elevating the staging of IA lung cancer with VPI to T2. Numerous studies have shown that VPI is an independent adverse prognostic factor for lung cancer, consistent with our research findings16,17,18. Vascular invasion and pleural visceral invasion are likely markers of tumor spread, indicating an increased risk of early recurrence in early-stage lung cancer. In this study, we found that the predominant tumor pattern, especially micropapillary and solid subtypes, had the significant impact on early recurrence in patients of stage IB LUAD, which was consistent with the previous studies12.Therefore, we included these risk factors in our analysis, significantly enhancing the accuracy of our predictive model for early recurrence in stage IB LUAD.
Currently, extensive research suggests a close association between early recurrence of malignant tumors and the body’s inflammatory response19,20. Neutrophils, as phagocytes, can promote tumor cell invasion and proliferation by releasing matrix metalloproteinase 9 (MMP-9) to degrade the extracellular matrix and by releasing vascular endothelial growth factor (VEGF)21. Platelets, derived from bone marrow megakaryocytes, form a physical barrier by adhering to tumor cells, protecting them from immune attack and enhancing their proliferation and invasion capabilities22. Lymphocytes regulate tumor growth by producing cytokines and are an essential component of the body’s immune response23. Decreased or depleted lymphocytes can weaken the anti-tumor immune response. Therefore, elevated NLR and PLR may promote tumor cell proliferation, invasion, and angiogenesis, leading to early recurrence and distant metastasis of malignant tumors. Wang et al. found a significant correlation between a high inflammatory response in the tumor microenvironment and immune suppression. A high inflammatory response was identified as an independent prognostic factor for early NSCLC after radical resection, correlating with poorer RFS and OS and serving as a predictive indicator for early recurrence of NSCLC24. This is consistent with the results of our study, that preoperative high NLR and PLR levels were identified as independent risk factors for early recurrence in patients with stage IB LUAD. Considering that a patient’s nutritional status may be related to the body’s anti-tumor immune capabilities, we included the prognostic nutritional index (PNI) in our analysis. Onodera, a Japanese scholar, first proposed the concept of PNI, which consists of preoperative serum albumin levels and peripheral blood lymphocyte counts25. Serum albumin reflects the body’s nutritional status, with malignant tumor patients often presenting with hypoalbuminemia. In recent years, numerous studies have shown that PNI is an independent factor affecting the prognosis of various solid tumors such as lung cancer, colorectal cancer, and breast cancer26,27,28. However, there is relatively limited research on the relationship between PNI and postoperative recurrence in early-stage NSCLC patients. In our study, we divided patients into two groups based on a PNI cutoff value of 48.3 and found that patients in the low PNI group had significantly lower RFS than those in the high PNI group. Therefore, preoperative PNI levels can serve as a predictive indicator for early recurrence in patients with stage IB LUAD. What’s more, some studies have shown that coagulation-related indices are also related to the prognosis of early lung cancer29. Xie et al. found thrombin time is a prognostic factor in postoperative patients with stage I-IIA. Coagulation-related indices have not been included in this study, and we will add coagulation-related indicess in subsequent studies to improve the accuracy of our model.
The need for postoperative adjuvant chemotherapy in patients with stage IB LUAD remains controversial, and guideline recommendations vary30. The NCCN recommends adjuvant therapy for high-risk patients with stage IB, where high-risk factors include poorly differentiated tumors, vascular invasion, wedge resection, and VPI, etc. However, the CSCO guidelines for the diagnosis and treatment of non-small cell lung cancer suggest that adjuvant chemotherapy is generally not recommended for LUAD at stage IB, including those with high risk factors, due to a lack of high-level evidence in support of adjuvant chemotherapy. In this study, a total of 105 patients (52.8%) received postoperative adjuvant chemotherapy, and the results showed that patients did not benefit from adjuvant chemotherapy, which may be due to the fact that we did not include postoperative receipt of targeted therapy in the analysis. Of the multiple known NSCLC driver mutations, EGFR is the predominant mutation type, accounting for approximately 45% of the mutations31.The ADAURA study showed that 3 years of adjuvant targeted therapy with oxitinib after complete tumor resection in patients with EGFR mutation-positive stage IB NSCLC reduces the risk of disease recurrence or death by 61% (HR = 0.39, 95%CI:0.18–0.88)32. Since the patients included in this study were operated before 2021 and the vast majority did not undergo postoperative genetic testing, we did not include postoperative targeted therapy in the analysis this time. This is a limitation of this study, and we will refine this section in subsequent studies.
In this study, we developed and validated a nomogram based on inflammation-nutrition indicators to predict the RFS of patients with stage IB LUAD. The nomogram demonstrated precise discriminative ability, offering valuable guidance for postoperative treatment decisions in patients with stage IB LUAD. Additionally, the accessibility of NLR, PLR, and PNI as clinical indicators significantly enhances the applicability of this predictive model in clinical practice. However, our study has several limitations. Firstly, it was a single-center retrospective study with a relatively small sample size, making it difficult to entirely avoid selection bias. And there was a lack of external validation of this study, which led to a lack of representativensess. Secondly, due to the absence of the data of genetic mutations, especially EGFR mutation, potentially impacting the accuracy of the predictive model. Thirdly, due to the follow up duration of this study was relatively short, the recurrence of patients with stage IB lung adenocarcinoma cannot be fully counted, which may impact the model’s predictive ability. Therefore, multicenter and prospective studies are needed to collect more informations and samples to validate our model in the future.
In summary, the inflammation-nutrition indices-based nomogram developed in this study has a certain predictive value for early recurrence in patients with stage IB LUAD.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
-
Sung, H. et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71(3), 209–249. https://doi.org/10.3322/caac.21660 (2021).
Google Scholar
-
Herbst, R. S., Morgensztern, D. & Boshoff, C. The biology and management of non-small cell lung cancer. Nature 24(7689), 446–454. https://doi.org/10.1038/nature25183 (2018).
Google Scholar
-
Goldstraw, P. et al. The IASLC Lung Cancer Staging Project: Proposals for revision of the TNM Stage groupings in the Forthcoming (Eighth) Edition of the TNM classification for Lung Cancer. J. Thorac. Oncol. 11(1), 39–51. https://doi.org/10.1016/j.jtho.2015.09.009 (2016).
Google Scholar
-
Nicholson, A. G. et al. The 2021 WHO classification of lung tumors: Impact of advances since 2015. J. Thorac. Oncol. 17(3), 362–387. https://doi.org/10.1016/j.jtho.2021.11.003 (2022).
Google Scholar
-
Lababede, O. & Meziane, M. A. The eighth edition of TNM staging of lung cancer: Reference chart and diagrams. Oncologist 23(7), 844–848. https://doi.org/10.1634/theoncologist.2017-0659 (2018).
Google Scholar
-
Ma, M. et al. Micropapillary or solid pattern predicts recurrence free survival benefit from adjuvant chemotherapy in patients with stage IB lung adenocarcinoma. J. Thorac. Dis. 10(9), 5384–5393. https://doi.org/10.21037/jtd.2018.08.64 (2018).
Google Scholar
-
Ruffini, E. et al. Significance of the presence of microscopic vascular invasion after complete resection of stage I-II pT1-T2N0 non-small cell lung cancer and its relation with T-Size categories: Did the 2009 7th edition of the TNM staging system miss something? J. Thorac. Oncol. 6(2), 319–326. https://doi.org/10.1097/JTO.0b013e3182011f70 (2011).
Google Scholar
-
Toyokawa, G. et al. Significance of spread through air spaces in resected pathological stage I lung adenocarcinoma. Ann. Thorac. Surg. 105(6), 1655–1663. https://doi.org/10.1016/j.athoracsur.2018.01.037 (2018).
Google Scholar
-
Zhu, J. et al. Development and validation of a new prognostic immune-inflammatory-nutritional score for predicting outcomes after curative resection for intrahepatic cholangiocarcinoma: A multicenter study. Front. Immunol. 14, 1165510. https://doi.org/10.3389/fimmu.2023.1165510 (2023).
Google Scholar
-
Iwai, N. et al. Neutrophil to lymphocyte ratio predicts prognosis in unresectable pancreatic cancer. Sci. Rep. 30(1), 18758. https://doi.org/10.1038/s41598-020-75745-8 (2020).
Google Scholar
-
Mandaliya, H., Jones, M., Oldmeadow, C. & Nordman, I. I. Prognostic biomarkers in stage IV non-small cell lung cancer (NSCLC): Neutrophil to lymphocyte ratio (NLR), lymphocyte to monocyte ratio (LMR), platelet to lymphocyte ratio (PLR) and advanced lung cancer inflammation index (ALI). Transl Lung Cancer Res. 8(6), 886–894. https://doi.org/10.21037/tlcr.2019.11.16 (2019).
Google Scholar
-
Qian, J-Y. et al. Prognostic evaluation of stage I lung adenocarcinoma based on systematic inflammatory response. JNCI Cancer Spectr. 7(6). https://doi.org/10.1093/jncics/pkad090 (2023).
-
Tsutani, Y. et al. High-risk factors for recurrence of stage I lung adenocarcinoma: Follow-up data from JCOG0201. Ann. Thorac. Surg. 108(5), 1484–1490. https://doi.org/10.1016/j.athoracsur.2019.05.080 (2019).
Google Scholar
-
Chen, Q. et al. Intratumoral and peritumoral radiomics nomograms for the preoperative prediction of lymphovascular invasion and overall survival in non-small cell lung cancer. Eur. Radiol. 33(2), 947–958. https://doi.org/10.1007/s00330-022-09109-3 (2023).
Google Scholar
-
Wang, S. et al. Proposal on incorporating lymphovascular invasion as a T-descriptor for stage I lung cancer. Lung Cancer 125, 245–252. https://doi.org/10.1016/j.lungcan.2018.09.024 (2018).
Google Scholar
-
Travis, W. D. et al. Visceral pleural invasion: Pathologic criteria and use of elastic stains: Proposal for the 7th edition of the TNM classification for lung cancer. J. Thorac. Oncol. 3(12), 1384–1390. https://doi.org/10.1097/JTO.0b013e31818e0d9f (2008).
Google Scholar
-
Jiang, L. et al. The impact of visceral pleural invasion in node-negative non-small cell lung cancer: A systematic review and meta-analysis. Chest 148(4), 903–911. https://doi.org/10.1378/chest.14-2765 (2015).
Google Scholar
-
Adachi, H. et al. Influence of visceral pleural invasion on survival in completely resected non-small-cell lung cancer. Eur. J. Cardiothorac. Surg. 48(5), 691–697. https://doi.org/10.1093/ejcts/ezu515 (2015). discussion 697.
Google Scholar
-
Trinchieri, G. Cancer and inflammation: An old intuition with rapidly evolving new concepts. Annu. Rev. Immunol. 30, 677–706. https://doi.org/10.1146/annurev-immunol-020711-075008 (2012).
Google Scholar
-
Tobias, D. K. et al. Markers of inflammation and incident breast Cancer risk in the women’s Health Study. Am. J. Epidemiol. 1(4), 705–716. https://doi.org/10.1093/aje/kwx250 (2018).
Google Scholar
-
Cristinziano, L. et al. Neutrophil extracellular traps in cancer. Semin Cancer Biol. 79, 91–104. https://doi.org/10.1016/j.semcancer.2021.07.011 (2022).
Google Scholar
-
Lazar, S. & Goldfinger, L. E. Platelets and extracellular vesicles and their cross talk with cancer. Blood 10(23), 3192–3200. https://doi.org/10.1182/blood.2019004119 (2021).
Google Scholar
-
Gutiérrez-Melo, N. & Baumjohann, D. T follicular helper cells in cancer. Trends Cancer 9(4), 309–325. https://doi.org/10.1016/j.trecan.2022.12.007 (2023).
Google Scholar
-
Wang, P. et al. Systemic inflammation influences the prognosis of patients with radically resected non-small cell lung cancer and correlates with the immunosuppressive microenvironment. Int. J. Cancer 15(4), 826–842. https://doi.org/10.1002/ijc.34547 (2023).
Google Scholar
-
Onodera, T., Goseki, N. & Kosaki, G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi 85(9), 1001–1005 (1984).
Google Scholar
-
Shoji, F. et al. Predictive impact for postoperative recurrence using the preoperative prognostic nutritional index in pathological stage I non-small cell lung cancer. Lung Cancer 98, 15–21. https://doi.org/10.1016/j.lungcan.2016.05.010 (2016).
Google Scholar
-
Zhang, X. et al. Preoperative prognostic nutrition index as a prognostic indicator of survival in elderly patients undergoing gastric cancer surgery. Cancer Manag. Res. 13, 5263–5273. https://doi.org/10.2147/cmar.S316437 (2021).
Google Scholar
-
Zhang, Y. et al. Predicting the prognosis of gastric cancer by albumin/globulin ratio and the prognostic nutritional index. Nutr. Cancer 72(4), 635–644. https://doi.org/10.1080/01635581.2019.1651347 (2020).
Google Scholar
-
Wu, L.-L. et al. Prognostic assessment of lung adenocarcinoma patients with early-staging diseases: A nomogram based on coagulation-related factors. Eur. J. Cardiothorac. Surg. 64(5). https://doi.org/10.1093/ejcts/ezad313 (2023).
-
Ettinger, D. S. et al. Non-small cell lung cancer, version 3.2022, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 20(5), 497–530. https://doi.org/10.6004/jnccn.2022.0025 (2022).
Google Scholar
-
Winfree, K. B. et al. Real-world characteristics and outcomes of advanced non-small-cell lung cancer patients with EGFR exon 19 deletions or exon 21 mutations. Future Oncol. 17(22), 2867–2881. https://doi.org/10.2217/fon-2021-0218 (2021).
Google Scholar
-
Wu, Y. L. et al. Osimertinib in resected EGFR-mutated non-small-cell lung cancer. N. Engl. J. Med. 29(18), 1711–1723. https://doi.org/10.1056/NEJMoa2027071 (2020).
Google Scholar
Acknowledgements
We would like to express our gratitude to our mentors and colleagues in the department for their guidance and support throughout this research and manuscript collaboration process.
Funding
Ningbo Clinical Research Center for thoracic & breast neoplasms (2021L002).
Author information
Authors and Affiliations
Contributions
X.H. write the main manuscript text. X.H. and Y.X. collect and analysis the data . X.H. and C. L. prepared figures. W.S. provided supervision for the research, critically reviewed and edited the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
Reprints and permissions
About this article
Cite this article
He, X., Xiang, Y., Lin, C. et al. Development and validation of an inflammation-nutrition indices-based nomogram for predicting early recurrence in patients with stage IB lung adenocarcinoma.
Sci Rep 14, 25111 (2024). https://doi.org/10.1038/s41598-024-76230-2
-
Received: 16 May 2024
-
Accepted: 11 October 2024
-
Published: 23 October 2024
-
DOI: https://doi.org/10.1038/s41598-024-76230-2
Keywords
- Stage IB lung adenocarcinoma
- Inflammation-nutrition indices
- Early recurrence
- Nomogram