Abstract
Background/Objectives
Understanding the impact of early-life nutritional choices on neurodevelopment in children is a growing area of research. To investigate the association between dietary patterns at multiple timelines and neurodevelopmental outcomes in 6-year-old children.
Subjects/Methods
This administrative observational study utilized a merged data from the national health insurance database and the health screening program for children. Information on the diet patterns from infancy to 3 years of age was obtained from parent-administered questionnaires. Dietary pattern clusters of the participants were identified using Polytomous Latent Class Analysis. The outcome was neurodevelopment using the Korean Developmental Screening Test (K-DST) at the age of 6 years.
Results
The study identified four distinct clusters among with the 133,243 eligible children (49.6% male, birth weight 3.22 kg, head circumference 42.7 cm at 4 months). The control cluster (53.4%) exhibited a diet including breast milk feeding and a variety of dietary patterns at the age of 1 year. In contrast, cluster 1 (36.0%) showed a skewed dietary pattern at the same age. Cluster 2 (6.6%) displayed diverse dietary patterns at one year but primarily consumed formula at four months, while cluster 3 (4.0%) had reduced dietary diversity and formula feeding. Compared with the control cluster, the adjusted odds ratio for unfavorable development was 1.209 (95% CI, 1.156–1.266) in cluster 1, 1.418 (95% CI, 1.312–1.532) in cluster 2, and 1.741 (95% CI, 1.593–1.903) in cluster 3. These findings remained consistent across individual domains of the K-DST.
Conclusions
Dietary patterns during infancy and early childhood may be associated with neurodevelopment at the age of 6 years.
Introduction
Neurodevelopmental growth and maturation occur rapidly during the fetal and infant periods. The brain exhibits a high degree of plasticity during this period [1]. Various factors influence cognitive development, including parent-child interaction, environmental complexity, parental socioeconomic status, nutritional support, and physical training [2,3,4]. The enriched environment has also been shown to impact neurogenesis, a factor affecting neural development significantly [5, 6]. An enriched environment includes various stimuli for physical activity, sufficient space, enough nutritional support, and physical training [7]. Additionally, enriched sensory and physical stimulation factors, such as nesting material, are also important [5, 8]. The mechanisms underlying the influence of enriched environments on brain development involve changes in the presentation of ion channels and neural circuits [9].
The type and variety of food can also play a role in creating an enriched environment. A restrictive diet is associated with early neurodevelopmental problems [10]. In an animal study, rats fed a soft diet showed negative effects on neurodevelopmental outcomes, particularly cell proliferation in the dentate gyrus of the hippocampus [11]. Similarly, a cross-sectional study conducted in China’s rural areas found that children who consumed a low number and variety of food items had delayed neurodevelopmental outcomes [12]. These findings demonstrate that feeding patterns during the neuro-vulnerable period of early infancy significantly influence neural development. Conversely, analyzing dietary patterns at a single time point during the postnatal period may pose challenges in evaluating developmental outcomes, as infant diets tend to change over time.
We hypothesize that dietary patterns during early childhood can influence neurodevelopment later in life. Therefore, this study aimed to investigate the effect of diverse weaning foods and diet patterns at multiple time points, utilizing clustering analysis utilizing cluster analysis and controlling for socioeconomic status, on children’s neurodevelopmental growth in six developmental domains. The findings from this study can be used to present appropriate guidelines for children’s eating habits to caregivers based on dietary patterns at various stages rather than at a single point in time.
Methods
Study design and data source
This study utilized a merged database from the National Health Insurance Service (NHIS) and the National Health Screening Program for Infants and Children (NHSPIC) in Korea. The NHIS is a single insurance system covering nearly the entire Korean population, making it a representative data source. The NHIS database provides baseline demographic characteristics, such as birth date, sex, insurance premium, and region of residence, as well as information on healthcare utilization, including the type of hospital visit, diagnosis codes (International Classification of Diseases 10th revision [ICD-10] codes), prescribed medication codes, and procedure codes. All children in Korea were eligible to undergo seven rounds of the NHSPIC, which were conducted at specific age intervals from four to 72 months of age. The rounds were scheduled as follows: 1st (4–6 months old), 2nd (9–12 months old), 3rd (18–24 months old), 4th (30–36 months old), 5th (42–48 months old), 6th (54–60 months old), and 7th (66–72 months old). The NHSPIC survey includes a general health questionnaire, the Korean Developmental Screening Test (K-DST), an anthropometric examination, and a physical examination [13].
The de-identified individual data were used only for research purposes. Patient consents were not required as this study was based on de-identified and publicly available data. The Institutional Review Board of the Korea National Institute for Bioethics Policy waived the need for informed consent. The study protocol was reviewed and approved by the Institutional Review Board of the Korea National Institute for Bioethics Policy (P01-201603-21-005). All methods were performed in accordance with the relevant guidelines and regulations.
Study population
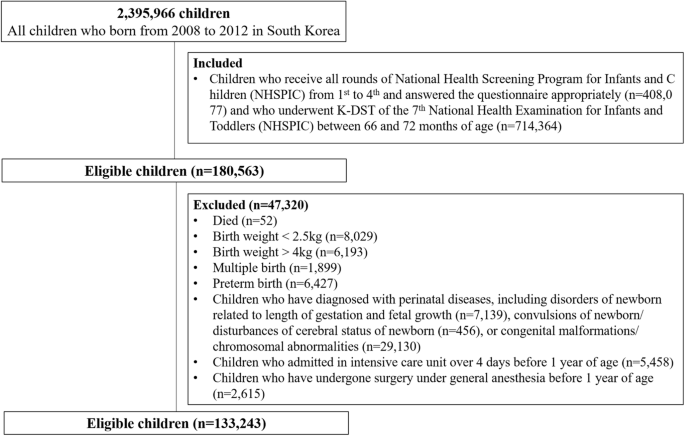
The study population is shown in Fig. 1. We included out of the 2,395,966 Korean children born between 2008 and 2012 those children who received all rounds of the NHSPIC from the first to the fourth round, responded appropriately to the questionnaire (n = 408,077) and received the K-DST properly in 7th round (n = 714,364). In total, 180,563 children met the inclusion criteria. Subsequently, children who met the following criteria were excluded: (1) died (n = 52), (2) birth weight <2.5 kg (n = 8029) or >4 kg (n = 6193), (3) multiple births (n = 1899), (4) preterm birth (n = 6427), (5) diagnosis with disorders of newborn related to the length of gestation and fetal growth (n = 7139), convulsions of newborn/disturbances of cerebral status of newborn (n = 456), or congenital malformations/chromosomal abnormalities (n = 29,130), (6) admission in intensive care unit over 4 days before 1 year of age (n = 5458), and (7) who received general anesthesia before 1 year of age (n = 2615) and for >5 days before 2 years of age were excluded. Ultimately, we enrolled 133,243 eligible children.

Study population.
Dietary patterns in young childhood
The information on dietary patterns from infancy to 3 years of age was provided from the NHSPIC questionnaire, spanning the first through fourth rounds. The details of the questionnaire were described in Supplementary Table 1. Specifically, the first round of the NHSPIC, conducted at the age of 4−6 months, includes questions about the types of milk infants consume. The second round, conducted at the age of 9−12 months, includes questions about the initiation time of the introduction complementary foods, the frequency of intake of complementary foods, and the ingredients included in complementary foods. The third round, conducted at the age of 18−24 months, included questions about the consumption frequency of fruit juice or sweetened beverages. Finally, the fourth survey, conducted at the age of 30−36 months, includes questions about the frequency of fruit juice or sweetened beverage consumption, meal frequency, and milk intake.
Clusters according to dietary patterns during young childhood
Polytomous Variable Latent Class Analysis (poLCA) was used to identify groups of similar cases within the manifest variables for dietary patterns during young childhood and determine whether they were statistically independent. We generated a series of models featuring a diverse range of latent clusters, spanning from two to ten. We evaluated the performance of each model to determine the optimal fit of the data and the greatest possible distinction between the identified clusters. We utilize several statistical measures to evaluate the quality of the model fit, including the maximum log-likelihood plot, which indicates the point at which the maximum log-likelihood ceases to increase significantly, and the elbow heuristic for the Bayesian Information Criterion (BIC) and Akaike Information Criterion (AIC), where the change in successive values becomes less noticeable. (Supplementary Table 3 and Supplementary Fig. 1) [14,15,16,17,18]. In addition, entropy values greater than 0.6 indicate good cluster separation [19, 20], and we considered the distribution of clusters acceptable when each cluster comprised more than 3% of the total participants. Based on the final model, four clusters were determined to provide the best fit.
Developmental status at preschool age
The preschoolers’ developmental status was assessed using the K-DST performed at the age of 66–72 months, which is a valid screening tool designed specifically for Korean children and is part of the NHSPIC inventory [21, 22]. The K-DST consists of six domains: gross motor, fine motor, cognition, language, sociality, and self-care. Each domain consisted of eight questions answered by a parent or legal guardian, and the results were interpreted in four stages. These stages were: advanced development (total score ≤1 standard deviation [SD] score), age-appropriate (total score ≥–1 SD score and <1 SD score), need for follow-up (total score ≥–2 SD score and <–1 SD score), and recommendations for further evaluation (total score <–2 SD score). Children whose results indicated the need for follow-up underwent retesting or further evaluation if the interviews indicated problems. If the results for any of the six domains indicated the need for follow-up or recommendations for further evaluation, the total K-DST score was considered to reflect the same. The outcome of interest was an unfavorable outcome of K-DST, defined as a result of a “need for follow-up” or “recommendation for further evaluation” in each domain or total score.
Covariates
Demographic variables such as sex, region at birth, economic status, and birth year were obtained from the NHIS database. The regions at birth were classified as Seoul, metropolitan, urban, or rural. Health insurance premiums were determined based on economic factors, including income level and assets. Therefore, the economic status was categorized into quintiles using health insurance premiums as the criteria for evaluation. Moreover, birth weight and head circumference at 4–6 months of age were considered baseline clinical variables and were obtained from the first round of the NHSPIC. In addition, diagnosed perinatal conditions, as baseline clinical variables, were observed using P-codes in ICD-10 codes. These condition included fetuses and newborns affected by maternal conditions, birth trauma, respiratory and cardiovascular disorders specific to the perinatal period, infections specific to the perinatal period, hemorrhagic and hematological disorders of the fetus and newborn, transitory endocrine and metabolic disorders specific to fetuses and newborns, digestive system disorders of the fetus and newborn, and conditions involving the integument and temperature regulation of the fetus and newborn. Furthermore, atopic dermatitis or food allergies, which can influence dietary habits, was assessed (details of disease definitions are provided in Supplementary Table 2).
Statistical analysis
Categorical variables are expressed as the total number (n) and percentage (%), and continuous variables are described as mean and SD. Categorical variables between clusters were compared using the chi-square test, and continuous variables were compared using the Student’s t test. A multivariate logistic regression model was used to calculate odds ratios (ORs) with 95% confidence intervals (CIs) to identify the associations between dietary patterns and unfavorable K-DST outcomes. In addition, interaction p value between ORs were calculated by comparing the logarithmic differences of the ORs. The standard errors of these differences were used to derive Z-scores, from which p values were obtained to assess statistical significance. All analyses were adjusted for sex, region at birth, economic status, calendar year at birth, birth weight, head circumference at 4–6 months of age, perinatal conditions, and comorbidities. All the analyses were performed using the poLCA package (ver. 1.6.0.1) of R package (ver. 4.1.3) and SAS version 9.4 (SAS Institute Inc, Cary, NC, USA). Two-sided P < 0.05 was considered statistically significant.
Results
Characteristics of clusters according to dietary pattern during young childhood
Of 133,243 eligible children, the study identified four distinct clusters: control, which included 71,169 children (53.4%); cluster 1, which included 47,990 children (36.0%); cluster 2, which included 8750 children (6.6%); and cluster 3, which included 5334 children (4.0%). The dietary patterns associated with each cluster are presented in Table 1.
The control cluster had the highest prevalence of human milk in infancy (51.6%), started weaning food between four and six months of age (85.8%), and provided most of the suggested ingredients in weaning foods approximately three times daily. In addition, at 2–3 years of age, more than two meals were provided (95.9%), juice intake was low, and 61.5% of children consumed 200–500 ml of milk daily.
Second, the Cluster 1had a higher rate of weaning initiation after 6 months of age than the control cluster (23.6 vs 12.2%). The most notable distinction between Cluster 1 and the other clusters was children’s selective consumption of weaned food. While the majority of children in this group included consumed grains, vegetables, and meat in their weaning diet (84.2%, 87.5%, and 95.7%, respectively), a relatively small proportion consumed fruits, eggs, and fish (53.3%, 22.5%, and 24.6%, respectively). Juice intake at 2 and 3 years of age was similar to that of the control cluster, with 94% of the children consuming 200 ml or less daily. In addition, the frequency of eating at around three years of age was two to three times, which accounted for most of the time (98.9%).
Third, the Cluster 2 had the lowest prevalence of human milk (22.6%) and was characterized by a late initiation of weaning food after 6 months of age (35.8%) despite the use of a variety of ingredients (87.8–96.9% of children consumed any type of solid food at 1 years of age). Compared to the control cluster, the ratio of children who drink sweetened juice 200 ml per day or more was high (4.3 vs 26.3% at the age of 2 years, 3.1 vs 28.8% at 3 years). Furthermore, almost 40% of the children under the age of three years had fewer than three meals daily.
Finally, in Cluster 3, exclusively human milk was used at a prevalence rate of 40.4%. The highest proportion was observed for introducing complementary feeding after six months (36.8%) among the four clusters. In contrast, the lowest proportion was observed for introducing all ingredients in complementary foods until 12 months of age (9.7–49.9%). At age 3, 85.4% of children ate more than three times daily, and 57.6% consumed between 200 to 500 ml of milk daily. Approximately 10% of the children consumed more than 200 ml of juice at 2 and 3 years of age.
Baseline characteristics of the study population
The baseline demographic and clinical characteristics of each cluster are presented in Table 2 and Supplementary Table 4. The study revealed that there were slightly more females than males in the control cluster (49.7% vs. 50.3%). The male-to-female ratios in the cluster 1 and 3 were similar to those in the control cluster. However, Cluster 2 showed the opposite trend, with a higher proportion of males than females (51.3 vs 48.7%, respectively). In addition, the four clusters differed in the region at birth and economic status (P < 0.01). The control cluster had a higher proportion of children born in Seoul and metropolitan areas and children in the highest quintile of economic status. In contrast, Cluster 3 had a higher proportion of children born in cities and rural areas and children from the lowest quintile of economic status.
The mean birth weight was 3.22 kg (SD, 0.33), with no significant differences among the groups, except for Cluster 3, which had a slightly lower mean birth weight (mean [SD], 3.21 [0.33]). In addition, there was no difference in head circumference at 4–6 months of age between the control cluster (mean [SD], 42.72 cm [1.45 cm]) and Cluster 2 (42.73 [1.48]), but cluster 1 and 3 (42.69 [1.46] and 42.61 [1.50], respectively) were slightly smaller than the those in the control cluster.
The association between the dietary pattern during infancy and young childhood and developmental status at preschool age
The associations between dietary patterns and developmental screening test results are shown in Table 3 and Fig. 2. Considering the control cluster as a reference, the results of the K-DST performed at six years of age were compared with those of the other clusters. The number of children with unfavorable outcomes according to the total score was 4448 (6.2%) in the control cluster, 3592 (7.5%) in Cluster 1, 794 (9.1%) in Cluster 2, and 586 (11.0%) in Cluster 3. The aOR for the unfavorable outcome of the total score was 1.209 (95% CI, 1.156–1.266) for Cluster 1, 1.418 (95% CI, 1.312–1.532) for Cluster 2, and 1.741 (95% CI, 1.593–1.903) for Cluster 3. This indicates that, compared to the control cluster, the impact on abnormal K-DST results at preschool age increases progressively from Cluster 1 to Cluster 2 and is strongest in Cluster 3 (all interaction p-values < 0.05).

Children were classified based on their dietary patterns in infancy and young childhood using polytomous variable latent class analysis. Adjusted odd ratios and their 95% confidence intervals were calculated by a multivariate logistic regression model with adjustment for sex, region at birth, economic status, birthweight, body weight at 4–6 and 9–12 months of age, head circumference at 4–6 months of age, perinatal conditions, and comorbidities. Filled squares indicate aOR and black lines indicate 95% CI. An asterisk indicates that the interaction p-value between two odds ratios is less than 0.05. aOR adjusted odd ratio.
Additionally, the associations between unfavorable outcomes and individual domains of the K-DST were consistent with the total score. Compared to the control cluster, the associations with unfavorable outcomes were significant across all individual domains for the other clusters, with Cluster 3 showing more than twice the strength of association with unfavorable outcomes compared to the control cluster.
Discussion
This national administrative and observational cohort study evaluated neurodevelopment at age 6 across various domains by categorizing children into four groups based on their dietary patterns during infancy and early childhood. Compared to a control cluster, the risk of unfavorable results in the neurodevelopmental screening test increased in the following order: Cluster 1, which involved selective ingredient use at at age 1; Cluster 2, characterized by high juice consumption; and Cluster 3, with a limited variety of ingredients at 1 year of age. It was confirmed that these differences in neurodevelopment could be applied to all domains of developmental screening tests, including gross motor, fine motor, cognition, language, sociality, and self-care. This study confirmed that dietary patterns in infants and young children are associated with neurodevelopment at 6 years of age.
The recommended dietary guidelines for infants and young children are as follows: Human milk feeding is encouraged during infancy [23], with the introduction of weaning foods typically beginning between 4 to 6 months of age [24]. Additionally, both infants and toddlers should be offered three main meals composed of a variety of ingredients [25, 26], along with a recommended milk intake of 200–400 ml [25, 27, 28]. The control cluster aligns well with these recommendations, characterized by a high rate of human milk consumption, the introduction of weaning foods between 4–6 months, a diverse range of food ingredients, and an appropriate meal frequency.
Compared to the control cluster, Cluster 1, 2, and 3 had a higher risk of abnormal outcomes of neurodevelopmental screening test by the K-DST at preschool age. Cluster 1 had slightly lower prevalence of human milk in infancy and a slightly higher proportion of children who initiated weaning food after 6 months. The most distinctive feature of Cluster 1 was picky eating regarding meal ingredients. While most children in this cluster were introduced to grains, vegetables, and meat, only a small proportion consumed fruit, eggs, and fish. In addition, Cluster 2 had the lowest prevalence of human milk consumption during infancy and the lowest proportion of children who ate three times a day at 1 and 3 years of age. Furthermore, Cluster 2 had the largest number of children among the four groups who consumed more than 200 ml of juice at ages 2 and 3. Cluster 3 had the highest proportion of children who initiated weaning after 6 months. The most distinctive feature of Cluster 3 was that many children were not introduced to ingredients such as fish, meat, eggs, or fruit in their diet at 1 year of age. The rate of consumption of grains or vegetables was less than half.
There are several potential mechanisms by which children with dietary patterns that deviate from the recommended guidelines in infancy and young childhood may experience negative impacts on neurodevelopment. First, the observed effects on neurodevelopmental outcomes may be linked to the role of an enriched environment, including feeding practices. The typical approach to implementing enriched environmental resources involves incorporating sensory and motor stimulation using various nest materials [29]. Animal experiments have demonstrated the positive effects of enriched environments on neurodevelopment [30, 31]. In addition, enriched resources have recently been recognized as relevant in the context of brain disorders [29, 32]. Additionally, enriched environments treat various developmental conditions in children, such as autism [33]. Sensory integration therapy typically involves visual, auditory, and tactile stimuli. Appropriate timing and various kinds of introduction of weaning food will be able to give these various gustatory stimuli, and it can be seen that this mechanism could be one of the important mechanisms in which the introduction of weaning food correlates with the development.
Second, Previous studies have investigated the impact of food on neurodevelopment from various perspectives, including nutrition [34, 35]. Assorted weaning food offers essential nutrients, including vitamins and minerals, crucial for physical growth and neurodevelopment. Studies have shown that growth during infancy, particularly in height and length, is associated with verbal and performance IQ in school-aged children [36]. Additionally, lower length measurements at 4 and 12 months were linked to lower cognitive function scores at 24 months [37]. As infants grow, their energy and nutrient requirements increase, necessitating additional nutrients such as iron, zinc, iodine, and various cofactors [38]. Micronutrient and caloric supplementation from complementary foods, especially in children of low socioeconomic status, promotes physical growth and neurodevelopment [10, 39]. The early growth patterns facilitated by introducing diverse weaning foods may contribute to improved brain development.
Last, the gut microbiome, influenced by diet and nutrition in children, significantly modulate brain function [40, 41]. Diet pattern influences gut microbiome via macronutrients and bioactive molecules and it also influences children’s attention [42]. In imaging studies, microbiome composition in infants affects early learning and cognitive scores at 2 years old without the differences in brain volume [43]. A longitudinal cohort study reported that specific microbiome patterns, dominantly including Genus Bacteroides were correlated with cognitive and language scores at children’s age 2 [44]. Children with autism spectrum disorder and cognitive and communication impairment have a specific pattern of microbiome and human transcriptome in age- and sex-matched cohort [45]. The body of evidence supporting the connection between the gut microbiome and neurodevelopment in early infancy and the relationship between the microbiome and brain development continues to expand.
This study had several notable strengths. First, it employed a longitudinal design with a large, representative sample of children, allowing for robust and generalizable findings. Second, poLCA use facilitated efficient observation of the effects of dietary patterns at different time points on children’s development through classification [46]. Thirdly, in contrast to previous research that primarily emphasized the benefits of human or fortified milk on children’s neurodevelopment, this study specifically focused on examining the impact of various dietary patterns during infancy and young childhood.
It is important to acknowledge the limitations of this study. First, information on dietary patterns was obtained from caregiver recall surveys, which introduced a potential recall bias. However, it is considered reliable because the survey asks about dietary patterns at various times. Additionally, it is challenging to ascertain comprehensive developmental outcomes based solely on the results of the K-DST performed at a single time point. Children exhibit individual variations in their developmental rates, and even those initially suspected of experiencing developmental demonstrate normal later often. Therefore, caution should be exercised when interpreting these results. Furthermore, disparities in the educational attainment of children and parents may exist despite the government’s provision of subsidies for preschool daycare centers. Moreover, factors related to the growth environment, such as siblings, family, and community, were not analyzed. These differences could be confounding variables; unfortunately, our dataset did not include this information.
In conclusion, we suggest that dietary practices during infancy and young childhood, including feeding type, timing of weaning food introduction, ingredients used in foods, frequency of intake, and the consumption of juice and milk, may influence neurodevelopment in preschool-aged children.
Data availability
This study was based on the National Health Claims Database established by the National Health Insurance Service of the Republic of Korea. Applications for using the National Health Insurance Service data are reviewed by the Inquiry Committee of Research Support; if the application is approved, raw data are provided to the applicant for a fee. We cannot provide access to the data, analytic methods, and research materials to other researchers because of the intellectual property rights of this database that is owned by the National Health Insurance Corporation. However, investigators who wish to reproduce our results or replicate the procedure can use the database, which is open for research purposes (https://nhiss.nhis.or.kr/ accessed on 7 July 2023).
References
-
Guyer AE, Perez-Edgar K, Crone EA. Opportunities for Neurodevelopmental Plasticity From Infancy Through Early Adulthood. Child Dev. 2018;89:687–97. https://doi.org/10.1111/cdev.13073.
-
Boesch C. Identifying animal complex cognition requires natural complexity. iScience. 2021;24:102195. https://doi.org/10.1016/j.isci.2021.102195.
-
Hackman DA, Farah MJ, Meaney MJ. Socioeconomic status and the brain: mechanistic insights from human and animal research. Nat Rev Neurosci. 2010;11:651–659. https://doi.org/10.1038/nrn2897.
-
Tooley UA, Bassett DS, Mackey AP. Environmental influences on the pace of brain development. Nat Rev Neurosci. 2021;22:372–384. https://doi.org/10.1038/s41583-021-00457-5.
-
Clemenson GD, Deng W, Gage FH. Environmental enrichment and neurogenesis: from mice to humans. Curr Opin Behav Sci. 2015;4:56–62.
Google Scholar
-
Kempermann G. Environmental enrichment, new neurons and the neurobiology of individuality. Nat Rev Neurosci. 2019;20:235–45. https://doi.org/10.1038/s41583-019-0120-x.
-
Zocher S, Schilling S, Grzyb AN, Adusumilli VS, Bogado Lopes J, Gunther S, et al. Early-life environmental enrichment generates persistent individualized behavior in mice. Sci Adv. 2020;6:eabb1478. https://doi.org/10.1126/sciadv.abb1478.
-
Sztainberg Y, Chen A. An environmental enrichment model for mice. Nat Protoc. 2010;5:1535–1539. https://doi.org/10.1038/nprot.2010.114
Google Scholar
-
Bayat M, Sharifi MD, Haghani M, Shabani M. Enriched environment improves synaptic plasticity and cognitive deficiency in chronic cerebral hypoperfused rats. Brain Res Bull. 2015;119:34–40. https://doi.org/10.1016/j.brainresbull.2015.10.001.
-
Anjos T, Altmae S, Emmett P, Tiemeier H, Closa-Monasterolo R, Luque V, et al. Nutrition and neurodevelopment in children: focus on NUTRIMENTHE project. Eur J Nutr. 2013;52:1825–1842. https://doi.org/10.1007/s00394-013-0560-4.
Google Scholar
-
Aoki H, Kimoto K, Hori N, Toyoda M. Cell proliferation in the dentate gyrus of rat hippocampus is inhibited by soft diet feeding. Gerontology. 2005;51:369–374. https://doi.org/10.1159/000088700.
-
Zhao C, Guan H, Shi H, Zhang J, Huang X, Wang X. Relationships between dietary diversity and early childhood developmental outcomes in rural China. Matern Child Nutr. 2021;17:e13073 https://doi.org/10.1111/mcn.13073.
Google Scholar
-
Kim JH, Lee JE, Shim SM, Ha EK, Yon DK, Kim OH, et al. Cohort profile: National Investigation of Birth Cohort in Korea study 2008 (NICKs-2008). Clin Exp Pediatr. 2021;64:480–488. https://doi.org/10.3345/cep.2020.01284.
Google Scholar
-
Hagenaars JA, McCutcheon AL. Applied latent class analysis. Cambridge University Press; 2002. https://doi.org/10.1017/CBO9780511499531.
-
Linzer DA, Lewis JB. poLCA: An R package for polytomous variable latent class analysis. J Stat Softw. 2011;42:1–29.
Google Scholar
-
Bandeen-Roche K, Miglioretti DL, Zeger SL, Rathouz PJ. Latent variable regression for multiple discrete outcomes. J Am Stat Assoc. 1997;92:1375–1386.
Google Scholar
-
Boehmke B, Greenwell BM. Hands-on machine learning with R. CRC Press, Boca Raton, FL, 2020. https://doi.org/10.1201/9780367816377.
-
Lezhnina O, Kismihók G. Latent Class Cluster Analysis: Selecting the number of clusters. MethodsX. 2022;9:101747.
Google Scholar
-
Tran T, Bliuc D, Ho-Le T, Abrahamsen B, van den Bergh JP, Chen W, et al. Association of Multimorbidity and Excess Mortality After Fractures Among Danish Adults. JAMA Netw Open. 2022;5:e2235856. https://doi.org/10.1001/jamanetworkopen.2022.35856.
Google Scholar
-
Asparouhov T, Muthén B. Auxiliary variables in mixture modeling: Three-step approaches using M plus. Struct Equ Model A Multidiscip J. 2014;21:329–341.
Google Scholar
-
Kim JH, Yi YY, Ha EK, Cha HR, Han MY, Baek HS. Neurodevelopment at 6 years of age in children with atopic dermatitis. Allergol Int. 2023;72:116–127. https://doi.org/10.1016/j.alit.2022.08.002.
Google Scholar
-
Kim D, Choe YJ, Durrani BAZ, Kim E, Byeon J, Eun BL. Korean Developmental Screening Test for Infants andChildren (K-DST): development, applications, and implications for future early childhood development interventions.Clin Exp Pediatr. 2023;66:288–293. https://doi.org/10.3345/cep.2022.00906
-
World Health Organization. Complementary feeding: report of the global consultation, and summary of guiding principles for complementary feeding of the breastfed child. In. Geneva: World Health Organization, 2003.
-
Fewtrell M, Bronsky J, Campoy C, Domellöf M, Embleton N, Mis NF, et al. Complementary feeding: a position paper by the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) Committee on Nutrition. J Pediatr Gastroenterol Nutr. 2017;64:119–132.
Google Scholar
-
U.S. Department of Health and Human Services and U.S. Department of Agriculture. 2015–2020 DietaryGuidelines for Americans. 8th Edition. 2015. Available at http://health.gov/dietaryguidelines/2015/guidelines/.
-
Hagan JF, Shaw JS, Duncan PM, eds. Bright futures guidelines for health supervision of infants, children, and adolescents, 4th ed. Elk Grove Village, IL: American Academy of Pediatrics, 2017. https://doi.org/10.1542/9781610020237.
-
Kim E, Chung S, Hwang J-T, Park YJ. 2020 Korean Dietary Reference Intakes for Protein: Estimation of protein requirements and the status of dietary protein intake in the Korean population. J Nutr Health. 2022;55:10–20.
Google Scholar
-
Guo Q, Wang B, Cao S, Jia C, Yu X, Zhao L, et al. Association between milk intake and childhood growth: results from a nationwide cross-sectional survey. Int J Obes. 2020;44:2194–2202.
Google Scholar
-
Bayne K. Environmental enrichment and mouse models: Current perspectives. Anim Models Exp Med. 2018;1:82–90.
Google Scholar
-
Brenes JC, Lackinger M, Hoglinger GU, Schratt G, Schwarting RK, Wohr M. Differential effects of social and physical environmental enrichment on brain plasticity, cognition, and ultrasonic communication in rats. J Comp Neurol. 2016;524:1586–1607. https://doi.org/10.1002/cne.23842. e-pub ahead of print 2015/07/02
Google Scholar
-
Chandler K, Dosso H, Simard S, Siddiqi S, Rudyk C, Salmaso N. Differential effects of short-term environmental enrichment in juvenile and adult mice. Neuroscience. 2020;429:23–32.
Google Scholar
-
Nithianantharajah J, Hannan AJ. Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat Rev Neurosci. 2006;7:697–709.
Google Scholar
-
Woo CC, Donnelly JH, Steinberg-Epstein R, Leon M. Environmental enrichment as a therapy for autism: A clinical trial replication and extension. Behav Neurosci. 2015;129:412.
Google Scholar
-
Krebs NF, Lozoff B, Georgieff MK. Neurodevelopment: The Impact of Nutrition and Inflammation During Infancy in Low-Resource Settings. Pediatrics. 2017;139:S50–S58. https://doi.org/10.1542/peds.2016-2828G.
-
Ha EK, Kim JH, Baek HS, Lee E, Baek JH, Shim S, et al. Association between complementary food introduction before age 4 months and body mass index at age 5-7 years: A retrospective population-based longitudinal cohort study. J Hum Nutr Diet. 2023;36:787–797. https://doi.org/10.1111/jhn.13098.
-
Pongcharoen T, Ramakrishnan U, DiGirolamo AM, Winichagoon P, Flores R, Singkhornard J, et al. Influence of prenatal and postnatal growth on intellectual functioning in school-aged children. Arch Pediatr Adolesc Med. 2012;166:411–416. https://doi.org/10.1001/archpediatrics.2011.1413.
-
Ramel SE, Demerath EW, Gray HL, Younge N, Boys C, Georgieff MK. The relationship of poor linear growth velocity with neonatal illness and two-year neurodevelopment in preterm infants. Neonatology. 2012;102:9–24. https://doi.org/10.1159/000336127.
-
Prado EL, Dewey KG. Nutrition and brain development in early life. Nutr Rev. 2014;72:267–284. https://doi.org/10.1111/nure.12102.
Google Scholar
-
Do BT, Hansen NI, Bann C, Lander RL, Goudar SS, Pasha O, et al. Associations between feeding practices and growth and neurodevelopmental outcomes at 36 months among children living in low- and low-middle income countries who participated in the BRAIN-HIT trial. BMC Nutr. 2018;4; https://doi.org/10.1186/s40795-018-0228-9.
-
Coley EJL, Hsiao EY. Malnutrition and the microbiome as modifiers of early neurodevelopment. Trends Neurosci. 2021;44:753–764. https://doi.org/10.1016/j.tins.2021.06.004.
Google Scholar
-
Lacorte E, Gervasi G, Bacigalupo I, Vanacore N, Raucci U, Parisi P. A Systematic Review of the Microbiome in Children With Neurodevelopmental Disorders. Front Neurol. 2019;10:727. https://doi.org/10.3389/fneur.2019.00727.
Google Scholar
-
Wang L-J, Yang C-Y, Chou W-J, Lee M-J, Chou M-C, Kuo H-C, et al. Gut microbiota and dietary patterns in children with attention-deficit/hyperactivity disorder. Eur child Adolesc psychiatry. 2020;29:287–297.
Google Scholar
-
Carlson AL, Xia K, Azcarate-Peril MA, Goldman BD, Ahn M, Styner MA, et al. Infant gut microbiome associated with cognitive development. Biol Psychiatry. 2018;83:148–159.
Google Scholar
-
Tamana SK, Tun HM, Konya T, Chari RS, Field CJ, Guttman DS, et al. Bacteroides-dominant gut microbiome of late infancy is associated with enhanced neurodevelopment. Gut Microbes. 2021;13:1930875.
Google Scholar
-
Morton JT, Jin D-M, Mills RH, Shao Y, Rahman G, McDonald D, et al. Multi-level analysis of the gut–brain axis shows autism spectrum disorder-associated molecular and microbial profiles. Nat Neurosci. 2023;26:1208–1217.
Google Scholar
-
Madigan C, Daley A, Kabir E, Aveyard P, Brown W. Cluster analysis of behavioural weight management strategies and associations with weight change in young women: a longitudinal analysis. Int J Obes. 2015;39:1601–1606.
Google Scholar
Funding
This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (grant number: HR22C1605-C). The funder has no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript
Author information
Authors and Affiliations
Contributions
Study concept and design: Dr. Rhie and Dr. Han. Drafting the manuscript: Dr. Rhie, Kim, and Han. Critical revision of the manuscript for important intellectual content: Dr. Kim, Dr. Ha, Dr. Shin, Dr. Han, and Dr. Rhie). Statistical analysis: Dr. Han, Miss Han, and Mr. Lee. Obtained funding: Dr. Rhie. Study supervision: Dr. Rhie and Han.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics Approval and consent to participate
The de-identified individual data were used only for research purposes therefore, patient consents were not required as this study was based on de-identified and publicly available data. The Institutional Review Board of the Korea National Institute for Bioethics Policy waived the need for informed consent. The study protocol was reviewed and approved by the Institutional Review Board of the Korea National Institute for Bioethics Policy (P01-201603-21-005).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
supplementary tables
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
Reprints and permissions
About this article
Cite this article
Kim, J.H., Ha, E.K., Lee, G.C. et al. Diverse weaning foods and diet patterns at multiple time points during infancy period and their association with neurodevelopmental outcomes in 6-year-old children.
Eur J Clin Nutr (2024). https://doi.org/10.1038/s41430-024-01528-3
-
Received: 22 February 2024
-
Revised: 10 October 2024
-
Accepted: 11 October 2024
-
Published: 18 October 2024
-
DOI: https://doi.org/10.1038/s41430-024-01528-3