Abstract
To test the hypothesis that dysregulated wound healing is associated with Urogynecologic mesh complications, we collected vaginal cell secretions using vaginal swabs after polypropylene mesh implantation in patients with (N = 39) and without (N = 40) complication. A customized multiplex immunoassay measured markers of inflammation (MCP-1, IGFBP-1, IL-2, IL-10, IL-17, PDGF-BB, bFGF, IL-1b, IL-6, IL-12p70, TNF-α), neuroinflammation (IL-1RA, TGF-β, IL-15, IL-18, IL-3, M-CSF), angiogenesis (VEGF), and matrix proteins (fibronectin, tenasin c, thrombospondin-2, lumican) between groups. Patients with complications were younger, heavier, implanted with mesh longer, and more likely to be ever smokers. A 5 kg/m2 BMI increase and ever-smoking were associated with a 2.4-fold and sixfold increased risk of complication, respectively. Patients with the highest tertile of bFGF, fibronectin, thrombospondin-2, TNF-β, or VEGF had an odds ratio (OR) of 11.8 for having a mesh complication while ≥ 3 elevated had an OR of 237 while controlling for age, BMI, and smoking. The highest tertile of bFGF, thrombospondin-2, and fibronectin together perfectly indicated a complication (P < 0.0001). A receiver-operator curve for high bFGF, thrombospondin-2, and fibronectin showed excellent discrimination between complications and controls (AUC 0.87). These data provide evidence of dysregulated wound healing in mesh complications. Modifiable factors provide potential targets for patient counseling and interventions.
Introduction
Pathologic fibrosis surrounding biomedical devices has long hindered the use of a variety of implants, including breast implants, insulin pumps, artificial joints, and surgical meshes1,2,3. The foreign body response, which in a well-integrated implant begins with inflammation and reaches homeostasis with a thin fibrotic capsule surrounding the implant, is a normal and expected tissue response4. However, complications can arise when the host tissue repair pathway after foreign body is implanted becomes dysregulated. These responses, particularly the development and control of fibrosis, are highly organ specific5.
Urogynecologic surgeons have used polypropylene meshes for decades to improve the durability of prolapse repairs and reduce invasiveness of surgery for incontinence, with more than 100,000 patients having prolapse mesh implanted annually6,7,8. However, even lightweight polypropylene meshes are associated with fibrosis-related pain and exposure, a condition in which the mesh shows through the vaginal epithelium in 4–10% of cases9,10,11. This can cause bleeding, dyspareunia for the patient and partner, and other complications12.
Healing after mesh implantation is complex and not only stimulates the foreign body response but also requires tissue ingrowth into the pores of the mesh. Like the acute foreign body response, the first stage of wound healing is a pro-inflammatory hemostatic response that seals the wound and prevents infection. The second stage is tissue replacement, a phase that drives fibroblast activation and differentiation into myofibroblasts via increased TGF-β. Myofibroblasts secrete collagen providing a provisional matrix that covers the wound and places traction on the wound edges. In the third and final phase known as the resolving phase, inflammation subsides and collagen maturation is completed. During this phase, myofibroblasts disappear from the wound in a wave of apoptosis. When wound healing becomes dysregulated, both chronic wound and scarring states ensue from the inability of the wound to enter a resolving phase13,14. Stark parallels exist between prolonged inflammation and high extracellular matrix turnover seen in mesh exposure complications and chronic skin wounds suggesting that mesh exposure may result from arrest in the inflammatory phase of wound healing13,15,16,17,18,19,20,21,22,23,24,25.
Likewise, the events observed in polypropylene mesh pain complications parallel dermal scarring. Increasing histologic fibrosis surrounding polypropylene vaginal mesh explants has been linearly associated with higher pelvic pain visual analog scores in women who had mesh removed for a mesh-related complication19. In addition, TGF-β, a profibrotic cytokine in many organ systems, was doubled in women who had persistent pain after mesh removal compared to those who improved after removal19. Despite the critical role of the extracellular matrix in wound healing and the promise it holds for therapeutic intervention26,27,28, very little is known about this process in vaginal biomaterial integration including mesh.
We hypothesized that patients with mesh complications will have evidence of abnormal wound healing. We further posited that proteins indicative of dysregulated extracellular matrix remodeling would be elevated in mesh complications, but that inflammatory markers would be similar between groups. To test this hypothesis, we aimed to assess a wide range of soluble markers of inflammation, pain and neuroinflammation, angiogenesis, and a hypothesis-driven set of matrix proteins critical for transition to the resolving phase of wound healing in patients with and without mesh complications. Clinical and demographic variables were collected and compared between the groups to identify factors that increase risk. As an exploratory aim, the same markers were compared in patients who reported improvement in pain after mesh removal to those who reported no improvement.
Methods
Patient selection
This study was approved by the University of Pittsburgh Institutional Review Board. All study procedures were performed in accordance with the relevant guidelines and regulations including STROBE guidelines. All patients presenting to the Magee Women’s Center for Bladder and Pelvic Health at the University of Pittsburgh who were scheduled to undergo a complete vaginal mesh excision for the complications of vaginal mesh exposure or mesh-related pain were offered enrollment in the Mesh Biorepository Cohort Study. Informed consent was obtained from all participants. Patients with mesh exposure into the urethra, bladder or rectum or clinical signs of infection (fever, elevated white blood cell count) were excluded. Age, BMI, gravity, parity, smoking status, menopausal status, use and type of hormone replacement therapy, comorbidities, variables describing the initial mesh placement and other demographic variables were collected at the time of enrollment using a standardized form developed a priori. Former smokers and current smokers were grouped together as “ever smokers.” Vaginal swabs were used to collect cells and secretions from the vaginal apex at the time of mesh removal. Complete vaginal mesh excisions were performed as described in the AUGS/IUGA Joint Statement on the Management of Mesh-Related Complications for the FPMRS Specialist12. Symptom questionnaires including visual analog scales (VAS) for pelvic pain scores and PFD-20 questionnaires were completed at enrollment and 6 months after removal. To meet the necessary sample size (see calculation below), samples were randomly selected from this biorepository.
For the control group, all patients with prior mesh placement (prolapse or incontinence mesh) at our institution without subsequent complications who were seen in the clinic for routine follow up beginning in 5/2019 were offered enrollment as controls. During the enrollment period, of the 84 patients invited to participate, 5 declined. Vaginal swabs from control patients were collected in the same way during routine pelvic examination until the necessary sample size was achieved (see calculation below).
Swab processing and pellet histology
After collection, swabs were placed into DPBS and frozen at − 80 degrees until all 79 samples were collected. Swabs were then thawed, vortexed, and centrifuged to remove cells and debris from the swabs. Slides were created from two test vaginal swabs prior to and after processing. These slides were stained with H&E to analyze cell type in the pellet and confirm removal of cells from the supernatant. Protein from the supernatant was then concentrated using centrifugation in commercial concentrator tubes (Thermo Scientific, Waltham, MA) until the protein concentration exceeded 2 mg/mL. Filtrate was confirmed to have an undetectable protein level indicating low protein loss.
Analyte quantification
A customized bead-based multiplex immunoassay (Luminex, R&D Systems, Minneapolis MN) was used to measure markers of inflammation or fibrosis (MCP-1, IGFBP-1, IL-2, IL-10, IL-17, PDGF-BB, bFGF, IL-1 β, IL-6, IL-12p70, TNF-α), pain and neuroinflammation (IL-1RA, TNF-β, IL-15, IL-18, IL-3, M-CSF), angiogenesis (VEGF), and matrix proteins critical for transition to resolving phase (fibronectin, tenasin c, thrombospondin 2, and lumican). Due to incompatibility with other analytes in the bead-based assay, TGF-β on 20 mesh and 15 control samples that had sufficient protein was analyzed separately using ELISA. An internal control was run on each plate.
Statistical analysis
Using previously published data on PDGF-BB19 from mesh explants and controls where 59% of mesh samples compared to 13% of controls were in the highest tertile with a corrected alpha of 0.0023 and a power of 90%, it was calculated that 38 samples per group (76 total) were needed to detect a difference. 79 total samples were collected to account for the potential for lost or insufficient samples and all of these were able to be used for analysis. Minimal plate variation was noted by comparing standard curves, however, to minimize plate-to-plate variation, data were normalized to an internal control that was run on all plates.
Demographic and clinical characteristics were compared between women with and without mesh complications using Student’s t-, Mann–Whitney U, and Fisher’s exact tests, where appropriate. Analytes below the detectable limit were replaced with lower limit of detection/2. Analytes were first analyzed as continuous variables and then significant variables were categorized into the highest tertile vs middle/low tertiles to assess the effect of high values of each analyte. The percentages of patients with the highest tertile of analyte were compared between groups using Fisher’s exact test with a Bonferroni correction (a1 = 0.0023). Logistic regression models containing analytes with P < 0.01 by univariate analysis assessed the odds of having a complication by analyte while controlling for age, BMI and smoking. Receiver-operator curves were generated for analytes that were different between groups. Fisher’s exact and Mann–Whitney U tests were performed to compare analytes in patients with complications who met the minimally important clinical difference of 13 mm improvement on a visual analog scale29 after removal (referred to as “responders”) as compared to those who did not. Receiver-operative curves were generated for these analytes.
Results
Patients with mesh complications were on average 9 years younger (56.0 ± 11.9 vs. 65.0 ± 8.9), heavier (BMI = 29.9 ± 5.5 vs. 26.6 ± 3.9) and more often ever smokers (58% or 23/40 vs. 28% or 11/39) than those without mesh complications (p < 0.05, Table 1). 87% of patients in the study were postmenopausal. Patients with mesh complications were more likely to be on both systemic and vaginal estrogen therapy (p < 0.05). They were also more likely to have received a mid-urethral sling as compared to a prolapse mesh and have a longer duration of implantation (p < 0.05, Table 1). The groups had similar rates of diabetes diagnosis. Each 5 kg/m2 BMI increase and ever smoking were associated with a 2.4-fold (p = 0.041) and sixfold (p = 0.026) increased risk of complication, respectively. When accounting for these variables, age was no longer a significant predictor of mesh complication.
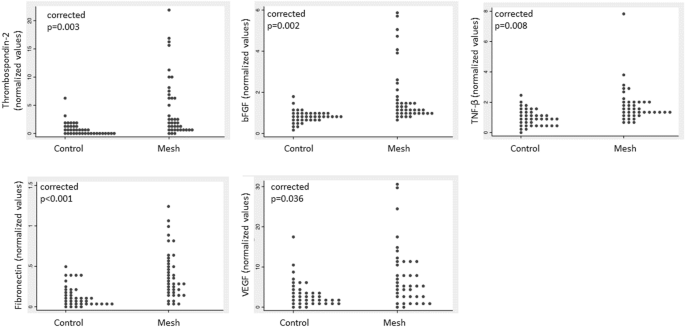
Patients with mesh complications had higher bFGF, fibronectin, thombospondin-2, TNF-β and VEGF than patients with no complications (Fig. 1, corrected p value < 0.05). No other analytes were statistically different between groups. When these 5 analytes were grouped into tertiles, more patients with mesh complications had the highest tertile of bFGF, fibronectin, thombospondin-2 (Table 2, corrected p value < 0.05). There were no differences in these analytes between sling and prolapse mesh complications (p > 0.05). Logistic regression models controlling for age, BMI and smoking status, demonstrated that swabs with high levels of any one of these analytes: bFGF, fibronectin, thrombospondin 2, TNF-β or VEGF had an odds ratio (OR) of 11.8 of having come from a patient with a complication. These odds increased further when 2 or 3 or more analytes were elevated (Fig. 2). The combination of high bFGF, and fibronectin and thrombospondin 2 perfectly indicated a complication swab (P < 0.0001) with a positive predictive value (PPV) of 100% and negative predictive value of 57%. When only sling mesh complications were included in the model, the same variables indicated a complication swab except for BMI, which was not different between slings with complications and slings without complications. There were no differences in analytes between pain and exposure complications.

Comparison of 5 significant analytes in patients with mesh complications compared to controls normalized to an internal control to minimize plate variation. P values are Bonferroni-corrected.

Predictors that the vaginal swab came from a patient with a complication.
A receiver-operator curve combining tertiles for bFGF, thrombospondin 2, and fibronectin showed excellent discrimination between complications and controls with an area under the curve of 0.87 (p < 0.001, Fig. 3).

Receiver-operator curve showing characteristics predictive that the swab came from a patient with a complication of high bFGF, high fibronectin and high thrombospondin 2 for mesh complications. The curve represents a score computed by assigning points to the tertile of each analyte of bFGF, thrombospondin 2, and fibronectin. The first tertile is 0 points, 2nd is 1 point and 3rd tertile is two points. So for example, a score of 0 indicates first tertile for all three analytes, a score of 5 indicates 3rd tertile of one analyte or 2nd tertile or two analytes while a score of 6 represents 3rd tertile for all three analytes. A score of 4 or 5 (which were equal) optimized sensitivity/specificity.
On our exploratory analysis of individuals who marked at least a 13 mm improvement on the pelvic pain VAS score after mesh removal (responders) compared to those who did not (non-responders) showed that TNF-β was lower in responders 1.28 (IQR 1.12–1.64; normalized values) compared to non-responders 1.64 (IQR 1.44–2.07; normalized values; P = 0.018). A receiver-operator curve to predict nonresponse based on TNF-β demonstrated an area under the curve of 0.70 (p = 0.027, Fig. 4). There were no differences in any other analyte studied between these two groups. Finally, there were no differences in analytes between patients with exposure complications (32/40, 80%) and those with pain in the absence of exposure (8/40, 20%).

Receiver-operator curve showing characteristics predictive that the swab came from a patient with a complication of highest tertile of bFGF, fibronectin, thrombospondin-2, TNF-β, and VEGF for improvement in pain after removal.
Discussion
In comparing soluble factors promoting inflammation, fibrosis, pain, or wound healing in vaginal swabs obtained from patients with Urogynecologic polypropylene mesh complications versus those with mesh implanted without complications, our most important findings were that patients with mesh complications were more likely to have high bFGF, fibronectin, thrombospondin 2, VEGF and TNF-β in vaginal secretions obtained in vaginal swabs at the time of mesh removal and these analytes were highly indicative of a complication compared to controls, which suggests an inability to switch to a mature, well-organized matrix related to the pathophysiology of the complication or an underlying disorder. In addition, we observed elevated TNF-β in patients who experienced poor improvement in pain after mesh removal and that patients with a history of smoking or increased BMI were associated with an increased odds of a complication.
Fibronectin and thrombospondin-2 are extracellular matrix proteins that regulate extracellular matrix homeostasis, and excess levels can contribute to fibrosis30,31. Specifically, fibronectin is increased in glomerular and interstitial fibrosis31,32 and thrombospondin-2 has recently been identified as a possible target for decreasing fibrosis following regenerative cardiac cell grafts33 as well as a biomarker for severe liver fibrosis34,35. Because the ECM is already altered in patients with prolapse36, this may represent a double-hit phenomenon for prolapse patients, where an already disorganized ECM with decreased mature type I collagen and increased type III collagen is unable to terminate the wound healing response when exposed to mesh. Further research into the subtypes and source (plasma or cellular) of fibronectin involved in mesh complications and its interactions with the ECM may prove a useful future direction.
VEGF and bFGF are signal proteins that act synergistically to induce new blood vessel formation after injury37. Clinically, neovascularization has also been reported within the mesh capsule during surgical removal that increases risk of bleeding38. New blood vessel formation accompanies fibroproliferation in wound healing and is an important contributor to fibrosis in some diseases, where fragile new blood vessels can break and contribute to repetitive injury and inflammation39,40 PDGF-BB, previously shown to be elevated in mesh complications19, and both PDGF-BB and TGF-β are upstream promoters of angiogenesis by activation of the STAT3 pathway, which upregulates VEGF and bFGF. Alternatively, VEGF and bFGF can be released directly from the ECM when it is degraded, as is seen in mesh exposure complications. bFGF is also mitogenic for fibroblasts as well as vascular endothelial cells41. Interestingly, in our prior work looking at tissue samples of patients with mesh complications compared to vaginal biopsy controls, bFGF was decreased which could be due to reciprocal inhibitory feedback with PDGF-BB and TGF-β19. Here elevated bFGF could indicate a broader profibrotic environment in the vagina which was detected in vaginal swabs compared to a very local downregulation in the tissue immediately adjacent to the mesh complication.
Proinflammatory TNF is produced by activated macrophages (TNF-α) and lymphocytes (TNF-β; conflicting data for TNF-α)42 and its receptors mediate pain sensitivity, likely through increased neuronal firing and increased inflammation43,44,45. Increased levels of TNF-α has been demonstrated in fibromyalgia46, mouse models of arthritis47, and centralization of pain48. Like TNF-α, TNF-β binds to both TNF receptors but may have different cytotoxicity and binding affinities42,49. TNF-β, but not TNF-α, has been shown to be elevated in vulvodynia50. The elevation of TNF-β in patients who did not respond to removal suggest neuropathic pain as a mechanism of persistent pain after mesh removal.
Demographic differences between groups such as age and duration of implantation may be affected by those who present for follow up and were therefore available as controls. Current smoking is an accepted risk factor for mesh complications51,52,53. There is conflicting data on obesity as a risk factor for mesh complications54,55, however, like smoking, obesity results in increased reactive oxygen species and inflammation, which may tip the organ system into dysregulated healing when a foreign body is introduced. Obesity also increases levels of circulating estrogens made in fat56 which likely softens the tissue and increases the stress mismatch with the highly stiff implant. Because midurethral slings are substantially stiffer than prolapse meshes this mismatch can also be greater with slings, which may account for our finding that more women in the complication group had midurethral slings. This mismatch may also be more pronounced in young women, in whom the vagina is softer, and who are at increased risk for complication57,58.
Injury, wound healing, and fibrosis progress differently in different organs such as the skin, heart, liver, and lungs. The closest analog to the vagina is often thought to be the skin or esophagus59, however the vagina is a unique organ with distinct structure, material properties, microbiome and inflammatory niche58. These data provide an important insight into dysregulated wound healing in mesh complications.
Strengths include a large study population and a novel control group. Tissue biopsy controls for mesh complications are difficult to obtain because women without complication generally do not have it removed and this has led to an inability to distinguish risk factors and pathophysiologic changes in some studies60. Therefore, using vaginal swabs from women with well-integrated mesh is novel and provides a unique look into mesh healing. In addition, we used a custom designed kit to test our hypothesis by looking specifically at regulatory ECM proteins important in wound healing in addition to traditional markers of inflammation. Our study has several notable limitations including that swabs collected at the time of mesh excision, as these were, do not provide prospective data to determine pre-implanation risk of mesh complication. In addition, mesh types may have a differential effect and we are unable to stratify by mesh type due to multiple products used in this patient population; however, the fact that the mesh products while variable in textile properties exhibit similar complications suggests that they likely share common pathophysiology. Although operative notes were reviewed when available, data on difficulty of insertion, vaginal atrophy, and immediate postoperative concerns such as hematoma were not available for most patients which can contribute to this multifactorial problem. Finally, cells and secretions on swabs may result in smaller levels of analytes although we have previously shown good concordance in protein levels between vaginal swabs and biopsies61.
Conclusions
In conclusion, high vaginal secretion levels of bFGF, thrombospondin 2, fibronectin, VEGF and TNF-β are strongly associated with ongoing mesh complications, with 3 elevated analytes having the strongest association. These data provide evidence of dysregulated wound healing in mesh complications. Further studies are needed to determine whether this plays a mechanistic role.
Data availability
Data is available upon request from the corresponding author.
References
-
Vieira, V. J. et al. Capsular contracture in silicone breast implants: Insights from rat models. An. Acad. Bras. Cienc. 88(3), 1459–1470 (2016).
Google Scholar
-
Kharbikar, B. N., Chendke, G. S. & Desai, T. A. Modulating the foreign body response of implants for diabetes treatment. Adv. Drug Deliv. Rev. 174, 87–113 (2021).
Google Scholar
-
Hori, R. Y. & Lewis, J. L. Mechanical properties of the fibrous tissue found at the bone-cement interface following total joint replacement. J. Biomed. Mater. Res. 16(6), 911–927 (1982).
Google Scholar
-
Carnicer-Lombarte, A., Chen, S.-T., Malliaras, G. G. & Barone, D. G. Foreign body reaction to implanted biomaterials and its impact in nerve neuroprosthetics. Front. Bioeng. Biotechnol. 15(9), 622524 (2021).
Google Scholar
-
Adusei, K. M., Ngo, T. B. & Sadtler, K. T lymphocytes as critical mediators in tissue regeneration, fibrosis, and the foreign body response. Acta Biomater. 1(133), 17–33 (2021).
Google Scholar
-
Kirby, A. C., Tan-Kim, J. & Nager, C. W. Midurethral slings: Which should I choose and what is the evidence for use?. Curr. Opin. Obstet. Gynecol. 27(5), 359–365 (2015).
Google Scholar
-
Chughtai, B. I. et al. Midurethral sling is the dominant procedure for female stress urinary incontinence: Analysis of case logs from certifying American Urologists. Urology 82(6), 1267–1271 (2013).
Google Scholar
-
Palmerola, R. et al. Trends in stress urinary incontinence surgery at a tertiary center: Midurethral sling use following the AUGS/SUFU position statement. Urology 131, 71–76 (2019).
Google Scholar
-
Dejene, S. Z., Funk, M. J., Pate, V. & Wu, J. M. Long-term outcomes after midurethral mesh sling surgery for stress urinary incontinence. Female Pelvic Med. Reconstr. Surg. 28(4), 188–193 (2022).
Google Scholar
-
Matthews, C. A. et al. Long-term mesh exposure after minimally invasive total hysterectomy and sacrocolpopexy. Int. Urogynecol. J. 34(1), 291–296 (2023).
Google Scholar
-
Page, A.-S. et al. Long-term data on graft-related complications after sacrocolpopexy with lightweight compared with heavier-weight mesh. Obstet. Gynecol. 141(1), 189–198 (2023).
Google Scholar
-
Developed by the Joint Writing Group of the American Urogynecologic Society and the International Urogynecological Association Rardin Charles R. Duckett Jonathan Milani Alfredo L. Paván Lucila Ines Rogo-Gupta Lisa Paván Lucila Ines Twiss Christian O. Joint position statement on the management of mesh-related complications for the FPMRS specialist. Female Pelvic Med. Reconstr. Surg. 26(4), 219–232 (2020).
Google Scholar
-
Wells, A., Nuschke, A. & Yates, C. C. Skin tissue repair: Matrix microenvironmental influences. Matrix Biol. 49, 25–36 (2016).
Google Scholar
-
Abramov, Y. et al. Histologic characterization of vaginal vs. abdominal surgical wound healing in a rabbit model. Wound Repair Regen 15(1), 80–86 (2007).
Google Scholar
-
Liang, R. et al. Extracellular matrix regenerative graft attenuates the negative impact of polypropylene prolapse mesh on vagina in rhesus macaque. Am. J. Obstet. Gynecol. 216(2), 153.e1-153.e9 (2017).
Google Scholar
-
Nolfi, A. L. et al. Host response to synthetic mesh in women with mesh complications. Am. J. Obstet. Gynecol. 215(2), 206.e1–8 (2016).
Google Scholar
-
Knight, K. M. et al. Mesh deformation: A mechanism underlying polypropylene prolapse mesh complications in vivo. Acta Biomater. 148, 323–335 (2022).
Google Scholar
-
Liang, R. et al. Towards rebuilding vaginal support utilizing an extracellular matrix bioscaffold. Acta Biomater. 15(57), 324–333 (2017).
Google Scholar
-
Artsen, A. M. et al. T regulatory cells and TGF-β1: Predictors of the host response in mesh complications. Acta Biomater. 115, 127–135 (2020).
Google Scholar
-
Brown, B. N. et al. Characterization of the host inflammatory response following implantation of prolapse mesh in rhesus macaque. Am. J. Obstet. Gynecol. 213(5), 668.e1–10 (2015).
Google Scholar
-
Liang, R., Knight, K., Abramowitch, S. & Moalli, P. A. Exploring the basic science of prolapse meshes. Curr. Opin. Obstet. Gynecol. 28(5), 413–419 (2016).
Google Scholar
-
Tennyson, L. et al. Characterization of the T-cell response to polypropylene mesh in women with complications. Am. J. Obstet. Gynecol. 220(2), 187.e1-187.e8 (2019).
Google Scholar
-
Junge, K. et al. Mesh biocompatibility: Effects of cellular inflammation and tissue remodelling. Langenbecks Arch Surg. 397(2), 255–270 (2012).
Google Scholar
-
Zhu, L.-M., Schuster, P. & Klinge, U. Mesh implants: An overview of crucial mesh parameters. World J. Gastrointest. Surg. 7(10), 226–236 (2015).
Google Scholar
-
de Almeida, S. H. M., Rodrigues, M. A. F., Gregório, E., Crespígio, J. & Moreira, H. A. Influence of sling material on inflammation and collagen deposit in an animal model. Int. J. Urol. 14(11), 1040–1043 (2007).
Google Scholar
-
Yates, C. C., Hebda, P. & Wells, A. Skin wound healing and scarring: Fetal wounds and regenerative restitution. Birth Defects Res. C Embryo Today 96(4), 325–333 (2012).
Google Scholar
-
Keane, T. J., Horejs, C.-M. & Stevens, M. M. Scarring vs. functional healing: Matrix-based strategies to regulate tissue repair. Adv. Drug Deliv. Rev. 6(129), 407–419 (2018).
Google Scholar
-
Xue, M. & Jackson, C. J. Extracellular matrix reorganization during wound healing and its impact on abnormal scarring. Adv. Wound Care (New Rochelle) 4(3), 119–136 (2015).
Google Scholar
-
Todd, K. H., Funk, K. G., Funk, J. P. & Bonacci, R. Clinical significance of reported changes in pain severity. Ann. Emerg. Med. 27(4), 485–489 (1996).
Google Scholar
-
Calabro, N. E. et al. Thrombospondin-2 regulates extracellular matrix production, LOX levels, and cross-linking via downregulation of miR-29. Matrix Biol. 82, 71–85 (2019).
Google Scholar
-
Patten, J. & Wang, K. Fibronectin in development and wound healing. Adv. Drug Deliv. Rev. 170, 353–368 (2021).
Google Scholar
-
To, W. S. & Midwood, K. S. Plasma and cellular fibronectin: Distinct and independent functions during tissue repair. Fibrogenesis Tissue Repair. 16(4), 21 (2011).
Google Scholar
-
Reinecke, H. et al. Lack of thrombospondin-2 reduces fibrosis and increases vascularity around cardiac cell grafts. Cardiovasc. Pathol. 22(1), 91–95 (2013).
Google Scholar
-
Kozumi, K. et al. Transcriptomics identify thrombospondin-2 as a biomarker for NASH and advanced liver fibrosis. Hepatology 74(5), 2452–2466 (2021).
Google Scholar
-
Adams, J. C. & Lawler, J. The thrombospondins. Cold Spring Harb Perspect. Biol. 3(10), a009712 (2011).
Google Scholar
-
Alarab, M., Kufaishi, H., Lye, S., Drutz, H. & Shynlova, O. Expression of extracellular matrix-remodeling proteins is altered in vaginal tissue of premenopausal women with severe pelvic organ prolapse. Reprod. Sci. 21(6), 704–715 (2014).
Google Scholar
-
Lieu, C., Heymach, J., Overman, M., Tran, H. & Kopetz, S. Beyond VEGF: Inhibition of the fibroblast growth factor pathway and antiangiogenesis. Clin. Cancer Res. 17(19), 6130–6139 (2011).
Google Scholar
-
Rogo-Gupta, L. & Castellanos, M. When and how to excise vaginal mesh. Curr. Opin. Obstet. Gynecol. 28(4), 311–315 (2016).
Google Scholar
-
Friedlander, M. Fibrosis and diseases of the eye. J. Clin. Invest. 117(3), 576–586 (2007).
Google Scholar
-
Wynn, T. A. Cellular and molecular mechanisms of fibrosis. J. Pathol. 214(2), 199–210 (2008).
Google Scholar
-
Laddha, A. P. & Kulkarni, Y. A. VEGF and FGF-2: Promising targets for the treatment of respiratory disorders. Respir. Med. 156, 33–46 (2019).
Google Scholar
-
Ruddle, N. Tumor necrosis factor (TNF-α) and lymphotoxin (TNF-β). Curr. Opin. Immunol. 4(3), 327–332 (1992).
Google Scholar
-
Li, J. et al. Combination of autophagy and NFE2L2/NRF2 activation as a treatment approach for neuropathic pain. Autophagy 17(12), 4062–4082 (2021).
Google Scholar
-
Dinarello, C. A. Historical insights into cytokines. Eur. J. Immunol. 37(Suppl 1), S34-45 (2007).
Google Scholar
-
Cook, A. D., Christensen, A. D., Tewari, D., McMahon, S. B. & Hamilton, J. A. Immune cytokines and their receptors in inflammatory pain. Trends Immunol. 39(3), 240–255 (2018).
Google Scholar
-
Theoharides, T. C., Tsilioni, I. & Bawazeer, M. Mast cells, neuroinflammation and pain in fibromyalgia syndrome. Front. Cell Neurosci. 2(13), 353 (2019).
Google Scholar
-
Richter, F. et al. Tumor necrosis factor causes persistent sensitization of joint nociceptors to mechanical stimuli in rats. Arthritis Rheum. 62(12), 3806–3814 (2010).
Google Scholar
-
Ji, R.-R., Nackley, A., Huh, Y., Terrando, N. & Maixner, W. Neuroinflammation and central sensitization in chronic and widespread pain. Anesthesiology 129(2), 343–366 (2018).
Google Scholar
-
Kircheis, R., Milleck, J., Korobko, V. G., Shingarova, L. N. & Schmidt, H. E. Differences in the biological activity of TNF alpha and TNF beta correlate with their different abilities for binding to the target cells. Eur. Cytokine Netw. 3(4), 381–390 (1992).
Google Scholar
-
Zanotta, N. et al. Cytokine profiles of women with vulvodynia: Identification of a panel of pro-inflammatory molecular targets. Eur. J. Obstet. Gynecol. Reprod. Biol. 226, 66–70 (2018).
Google Scholar
-
Kokanali, M. K. et al. Risk factors for mesh erosion after vaginal sling procedures for urinary incontinence. Eur. J. Obstet. Gynecol. Reprod. Biol. 177, 146–150 (2014).
Google Scholar
-
Deng, T., Liao, B., Luo, D., Shen, H. & Wang, K. Risk factors for mesh erosion after female pelvic floor reconstructive surgery: A systematic review and meta-analysis. BJU Int. 117(2), 323–343 (2016).
Google Scholar
-
Withagen, M. I., Vierhout, M. E., Hendriks, J. C., Kluivers, K. B. & Milani, A. L. Risk factors for exposure, pain, and dyspareunia after tension-free vaginal mesh procedure. Obstet. Gynecol. 118(3), 629–636 (2011).
Google Scholar
-
Wen, Q. et al. Impact of obesity on operative complications and outcome after sacrocolpopexy: A systematic review and meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 258, 309–316 (2021).
Google Scholar
-
Wan, O. Y., Chan, S. S., Cheung, R. Y. & Chung, T. K. Mesh-related complications from reconstructive surgery for pelvic organ prolapse in Chinese patients in Hong Kong. Hong Kong Med. J. 24(4), 369–377 (2018).
Google Scholar
-
Mair, K. M., Gaw, R. & MacLean, M. R. Obesity, estrogens and adipose tissue dysfunction – Implications for pulmonary arterial hypertension. Pulm Circ. 10(3), 2045894020952019 (2020).
Google Scholar
-
Kelly, E. C., Winick-Ng, J. & Welk, B. Surgeon experience and complications of transvaginal prolapse mesh. Obstet. Gynecol. 128(1), 65–72 (2016).
Google Scholar
-
Wira, C. R., Fahey, J. V., Sentman, C. L., Pioli, P. A. & Shen, L. Innate and adaptive immunity in female genital tract: Cellular responses and interactions. Immunol. Rev. 206, 306–335 (2005).
Google Scholar
-
Uhlén, M. et al. Tissue-based map of the human proteome. Science 347(6220), 1260419 (2015).
Google Scholar
-
Khrucharoen, U. et al. Clinical predictors and risk factors for vaginal mesh extrusion. World J. Urol. 36(2), 299–304 (2018).
Google Scholar
-
Oliphant, S. S., Nygaard, I. E., Zong, W., Canavan, T. P. & Moalli, P. A. Maternal adaptations in preparation for parturition predict uncomplicated spontaneous delivery outcome. Am. J. Obstet. Gynecol. 211(6), 630.e1–7 (2014).
Google Scholar
Funding
This work is funded by the NIH/ORWH Building Interdisciplinary Research Careers in Women’s Health (BIRCWH) NIH K12HD043441 scholar funds to AA and NIH RO1 HD083383-01. NIH R21HD099549, NIH R01HD108666, PA DOH Tobacco Grant.
Author information
Authors and Affiliations
Contributions
A.A. and P.M. contributed to study design, sample collection, data collection, data analysis and interpretation and wrote the manuscript. R.L and L.M contributed to data analysis and interpretation. AA and L.M. created the figures. M.B. contributed to sample collection. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
Reprints and Permissions
About this article
Cite this article
Artsen, A.M., Liang, R., Meyn, L. et al. Dysregulated wound healing in the pathogenesis of urogynecologic mesh complications.
Sci Rep 13, 21437 (2023). https://doi.org/10.1038/s41598-023-48388-8
-
Received: 28 April 2023
-
Accepted: 26 November 2023
-
Published: 05 December 2023
-
DOI: https://doi.org/10.1038/s41598-023-48388-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.