Abstract
The body condition of a snake species provides important physiological, morphological, and ecological information that elucidates its habits, life cycle, and competitive relationships. We measured the body size and condition of the wild Gloydius ussuriensis population in South Korea from 2018 to 2022, analyzed the degree of intraspecific niche overlap, and identified the geographic and climatic factors affecting their body condition. We found that the females were longer than the males. The body condition index (BCI) of G. ussuriensis differed depending on sex and season; the BCI of the females and males was highest in August and October, respectively. Environmental factors related to altitude and temperature affected the body condition of G. ussuriensis; BCI increased as the mean annual temperature and winter temperature increased; however, it increased when the annual temperature range decreased. The mean Pinaka index was 0.96, indicating a high degree of niche overlap; however, the niche overlap among the neonates was less than that among the adults and juveniles. To elucidate the causes of niche overlap and mechanisms behind the intraspecific competition among G. ussuriensis individuals, the habitat and utilization of food resources at different development stages of G. ussuriensis should be further investigated.
Introduction
Importance of research on body condition
The body size of an animal is closely related to its home range1, movement speed2, body condition3, and energy requirement4. Information about the body size of an animal is important in animal physiology, morphology, and ecology because it elucidates the habits, life cycle, and competitive relationships of an animal5,6,7. Snakes belong to class Reptilia and are characterized by their unique morphological traits: long body shape and absence of limbs. Instead of using weight, scientists use snout–vent length (SVL), which is less affected by breeding, season, and feeding changes, to measure body size. The slope of weight versus SVL can be used to examine animal habits and compare the body shape of different species2,8,9. Thus, information about the weight and length of a snake is important in determining its body condition. Body conditions can be used to identify the breeding and feeding periods of an animal species and determine the health and well-being of each individual2,3. Various methods have been proposed for calculating the body condition index (BCI); a method that more accurately reflects the fat and protein stores of an animal was developed by standardizing the body size of an animal regardless of its growth, age, and sex10,11,12. The BCI has been employed in various ecological studies on snakes, including the conservation of endangered species, control of invasive species, and understanding of evolutionary body shapes and behavioral habits2,3,13.
Niches and intraspecific competition among snakes
Snakes occupy a specific ecological niche within their environment for survival and breeding. Information about niches is useful for understanding the biogeographical range and evolution of species14,15. Niches can be broadly quantified along the axes of food, space, and time; such a quantification allows the analysis of intra- and interspecific niche overlap and niche separation16,17. Snakes have a high ecological and morphological variation not only between species but also between individuals (i.e., males and females; adults and juveniles) 9,18,19. Niche overlap applies a strong selective pressure on animals, causing a reduced or unequal allocation of resources due to intraspecific competition, which ultimately leads to regional population decline or extinction due to reduced fecundity20. Therefore, research on intraspecific niches plays an important role in conserving ecological niches and understanding competition among the individuals of the same species1,21,22.
Gloydius ussuriensis
Gloydius ussuriensis has a broad range spanning Korea, China, and Russia. It has been classified as an endangered species in China and is designated as a protected species (no capture) in Korea to prevent its population from declining due to poaching23,24,25. In Korea, G. ussuriensis is widely distributed across the entire inland peninsula as well as in Jeju-do and other islands of various areas. It usually inhabits rivers, valleys, and agricultural land adjacent to mountainous regions at an altitude of 0–1000 m15,26,27. It is mainly active from April to October and hibernates from November until March of the succeeding year25. Females can store sperm for a long time, are ovoviviparous, and produce 3–10 offspring between August and September; however, they do not produce offspring annually28. Numerous studies focusing on the different aspects of G. ussuriensis, including studies on morphology23,28,29,30, genetic diversity31,32, sexual dimorphism of island populations33, food sources34,35, toxicity36, intraspecific competition15,17, and conservation37, have been conducted. However, studies focusing on the body condition of and intraspecific competition among G. ussuriensis individuals have not yet been conducted.
Objectives
We conducted this study to identify the general trends in a G. ussuriensis in Korea. We measured and analyzed the body shape of G. ussuriensis, examine its behavioral habits and life cycle, identify the major environmental factors affecting its body condition, and calculate the intraspecific niche overlap. To attain these objectives, we measured differences in body size depending on age and sex, calculated the BCI of the males and females, and compared the results with the month when the measurements were taken. We also determined the environmental factors—geographic and climatic—in the regions where G. ussuriensis is commonly found, analyzed the effects of the major environmental factors on its body condition, and determined the occurrence of intraspecific competition based on the extent of niche overlap by age and sex.
Results
Snake capture
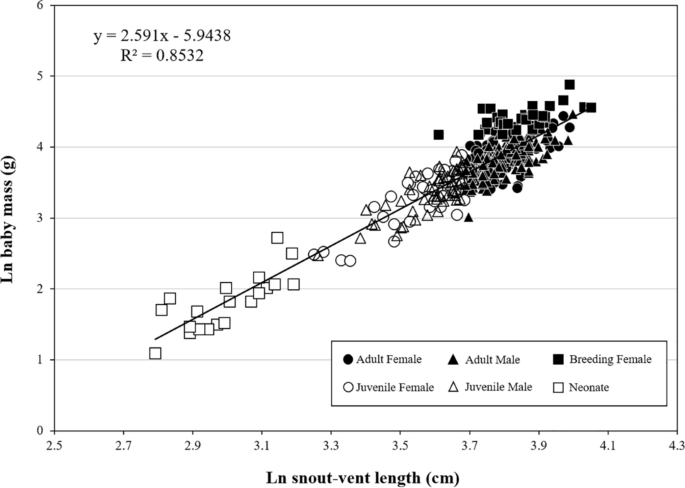
A total of 356 G. ussuriensis specimens were captured, of which 245 were adults (female: 135, male: 110), 91 were juveniles (female: 38, male: 53), and 20 were neonates (Table 1). There were 32 pregnant females (adult: 31, juvenile: 1; Fig. 1).
Body size
The regression equation for ln M on ln SVL was ln M = 2.591 ± 0.057 ln SVL – 5.944 ± 0.211 (r2 = 0.853, F(1,354) = 2056.9, p < 0.0001, Fig. 1; Table 2). In terms of body size, the adult females (n = 135) had longer SVL than the males [n = 110; 44.89 ± 0.32 cm vs. 44.71 ± 0.32 cm, F(1,243) = 10.94, p < 0.0001] and higher mass-to-SVL ratio [1.24 ± 0.03 g/cm vs. 1.03 ± 0.02 g/cm, F(1,243) = 163.30, p < 0.0001; Table 3]. The juvenile females (n = 38) did not differ with the males (n = 53) in either SVL [35.46 ± 0.61 cm vs. 36.35 ± 0.43 cm, F(1,89) = 1.495, p > 0.05] or mass-to-SVL ratio [0.82 ± 0.04 g/cm vs. 0.81 ± 0.03 g/cm, F(1,89) = 0.05, p > 0.05; Table 2].
Body condition
Body condition had a significant sex [F(1,334) = 27.02, p < 0.001] and month [F(1,334) = 6.66, p < 0.01], whereas interaction of sex and month had not a significant [F(1,334) = 2.56, p = 0.110]. The BCI of the females in August was higher than that in April (p < 0.01), May (p < 0.01), September (p = 0.01), and October (p < 0.05; Fig. 3); the BCI of the females in April was lower than that in June (p < 0.05) and July (p < 0.05; Fig. 3). The BCI of the males in October was higher than that in April (p = 0.01), May (p < 0.001), June (p < 0.01), July (p < 0.001), August (p < 0.01), and September (p < 0.01); the BCI of the males in May was lower than that in August (p < 0.05) and September (p < 0.05; Fig. 3). The BCI of the females was higher than that of the males in May (p < 0.05), June (p < 0.05), July (p < 0.01), and August (p < 0.001; Fig. 2).
Correlations between climatic factors
When we performed PCA using the climate variables at the locations where G. ussuriensis was observed, there were three PCs with eigenvalues > 1, and these explained 92.05% of the variance (Table 3). PC1 best explained the variance in the mean annual temperature, winter temperature, and annual temperature range. When PC1 increased, the mean annual temperature and winter temperature increased; however, the annual temperature range decreased. PC2 was mostly correlated with precipitation-related variables. When PC2 increased, the mean annual precipitation, summer precipitation, and winter precipitation increased. PC3 mostly explained summer temperature, which increased as PC3 increased (Table 3).
Variables affecting body condition, body mass (g), and SVL (cm)
We found that the environmental factors affecting G. ussuriensis body condition were altitude, PC1, and PC3 and body mass and SVL were altitude, PC1, PC2, and PC3 (Table 6). The BCI of G. ussuriensis increased as PC1 increased (Fig. 3).
Intraspecific niche
The degree of intraspecific niche overlap was extremely high; the probability densities measured for altitude and the three PCs revealed a high degree of overlap (Fig. 4). These results were reflected in the PI, which is a measure of ecological niches based on geographic variables (Table 4). The intraspecific PI was highest for habitat (PI: 0.994; p < 0.001), followed by PC3 (PI: 0.973; p < 0.001), altitude (PI: 0.971; p < 0.001), PC2 (0.957; p < 0.001), and PC1 (0.886; p < 0.01; Table 6). There was a high degree of overlap between the juvenile females and males for altitude, habitat, PC1, and PC2; in contrast, there was a low degree of overlap between the juveniles (both male and female) and neonates for the abovementioned variables (Table 4). For PC3, the niche overlap between the juvenile females and neonates was the highest, while that between the adult and juvenile males was the lowest (Table 4).
Discussion
In this study, we measured and compared the body size and condition of G. ussuriensis to describe their life cycle and behavioral habits. We also determined the effects of geographic and climatic factors in the habitat of G. ussuriensis on its body condition and analyzed the degree of intraspecific niche overlap. The females were longer than the males; the SSD index was 0.004, suggesting that female snakes evolved to be larger than males to improve their reproductive ability. BCI differed depending on sex and month; the females had the highest BCI in August, which is the reproductive season; meanwhile, the males had the highest BCI in October, which is just before hibernation. The environmental factors affecting the body condition of G. ussuriensis were the climatic factors PC1 and PC3 and the geographic factor altitude. When PC1 increased, the body condition of G. ussuriensis also increased. The mean PI was 0.96, indicating a very high degree of niche overlap according to age and sex.
Body size
The slope of body size versus weight for G. ussuriensis was 2.59, which is similar to the mean slope (2.67) derived from 60 Viperidae species, was within the 95% confidence interval. Moreover, the slope value of 2.59 is also similar to the interspecific allometric slope of 2.52 derived from 166 Colubridae species2,38. Generally, snakes that have thicker bodies are less active in foraging than those with wider bodies and are more likely to adopt a feeding strategy of hiding and waiting for prey9,39,40. Viperidae species are specialized for ambush hunting41,42. Snake species that utilize various food sources move more actively and ambush or hunt less frequently than those with less diverse food sources43. For example, among 23 Bothrops species (family Viperidae), the two species with the most diverse food sources (6 taxa; B. atrox and B. jararaca) seek locations with the most abundant food and forage actively; however, they also carry out ambush hunting occasionally40,44. The G. ussuriensis population in Korea utilize diverse food sources; their main food sources are amphibians and rodents, followed by fish, mammals, reptiles, and invertebrates. On the other hand, G. intermedius only feeds on rodents26,34,45. According to previous research that utilized wireless tracking devices to study the G. ussuriensis population in Korea, the mean travel distance of G. ussuriensis (39.64 ± 22.11 m), which moved for 1 month (September), was longer than that of G. saxatilis (21.50 ± 23.40 m), which moved for 2 months (August and September46,47). In particular, G. intermedius is a stationary species, and the frequency of its ambush and hiding reached 92.69% (active 6 times out of 82 observations48). Gloydius ussuriensis has a thinner body than other Viperidae species and utilize more diverse food sources; hence, it actively forages for prey.
Sexual size dimorphism
Although SSD index of our G. ussuriensis was 0.004, which is a very low value, the fact that females have evolved to be larger than males is meaningful in evolutionary ecology (Table 4). Typically, female snakes require more intra-abdominal space to allow the growth of eggs and embryos; therefore, they have evolved to be larger than males to improve their reproductive ability9,20,38. Viperidae species are an exception because the males are typically larger than the females. Previous studies have reported that there are only 6 out of 29 Viperidae species in which the females are larger than the males; these species have an SSD index ranging from 0.01 to 0.152,9. Although Korean G. ussuriensis females are larger than males, the difference between the two sexes is minimal, unlike in other Viperidae species. Meanwhile, different SSD indices were computed for the G. ussuriensis populations in islands such as Jeju-do ((-) 0.03) and Gapa-do (0.02)33. Such a difference in SSD indices is attributed to the differential evolution of the island populations due to the ecological and geographic characteristics of islands, where environmental factors (food, habitats, climate, etc.) are typically restricted28. For example, the main food sources of G. ussuriensis, which are amphibians (inland: 23 species, Jeju: 7 species) and rodents (inland: 19 species, Jeju: 8 species), geographically differ in terms of species diversity. Since these animals are more diverse in inland regions than in Jeju-do, there are also differences in the preferred food sources between the inland and Jeju-do populations of G. ussuriensis26,27,35,49. In the G. ussuriensis population in Jeju-do, the males have evolved to be larger than the females due to intrasex competition, as a means of increasing the frequency of mating and occupying favorable breeding grounds; these species also tend to show a higher rate of male-to-male combat behavior21,50. Nevertheless, combat behavior among male G. ussuriensis has not yet been documented28,31,51. According to previous studies, carpet pythons (Morelia spilotus) exhibit differences in combat behavior depending on the subspecies (M. s. spilotus vs M. s. variegatus52,53). Hence, further research should be conducted to determine the unknown combat behaviors, in addition to the typical ones (e.g., head raising, dancing, etc.), of adult G. ussuriensis males9. In summary, the G. ussuriensis females in Korea were larger than the males, and it is rare among Viperidae species to have a positive SSD index.
Body condition
The BCI of G. ussuriensis was lowest in April for both males and females and was highest in August and October for females and males, respectively. The reproductive cycle influences the BCI of G. ussuriensis females: they form yolks in May, are close to parturition in August (the month when the highest BCI was recorded), and give birth in September28,54. Thus, we observed that the females had higher BCI than males between May and August; however, their BCI decreased rapidly after they had given birth in September. Generally, the body condition of female snakes rapidly decline immediately after giving birth, and 1–4 years are required for them to fully replenish their energy2,55. On the other hand, the reproductive cycle has different effects on the body condition of male snakes depending on the species2,7. For example, there is no difference in body condition among asp viper (Vipera aspis) males during the reproductive season in spite of high testosterone levels and courtship behaviors, while the BCI of pygmy rattlesnake (Sistrurus miliarius) males increases as testosterone levels increase7,56. The testosterone levels of G. ussuriensis males begin to increase in May, peak in August, and rapidly decline at the end of September54. The body condition of the G. ussuriensis males did not differ from May to August, suggesting that they are unaffected by the reproductive season. Unlike females, which require a considerable amount of energy during pregnancy and parturition, males utilize less energy for reproduction; thus, they are able to maintain a higher body condition18,56.
It is hypothesized that the males had the highest BCI in October because they would like to increase their survival rate during hibernation. During the long hibernation period, snakes typically live in a harsh environment where they are unable to forage; such harsh living conditions have negative effects on their body condition and survival[57.58]. For example, a study that investigated the weight loss and mortality of 98 snake specimens from three species during hibernation and post-hibernation revealed that the striped whipsnake (Masticophis taeniatus) had 39% mortality rate and a 9.4% decrease in body weight, the Great Basin rattlesnake (Crotalus lutosus) had 34% mortality rate and a 7.6% decrease in body weight, and the Western yellow-bellied racer (Coluber constrictor mormon) had 50% mortality rate and an 11% decrease in body weight57. Meanwhile, a 3-year experimental study of 950 northern water snake (Nerodia sipedon) neonates revealed that their survival rate during hibernation was only 47%58. For this reason, snakes must feed as much as possible before hibernating to increase their body condition and improve their survival rate during hibernation59. This explanation supports our finding that the BCI of the males was higher in October, which is the time before hibernation, than in the other months. In summary, changes in the body condition of G. ussuriensis appropriately reflect their life cycle, including reproduction, hibernation, and feeding behaviors. The BCI of the females and males was lowest in April, when they had woken from hibernation. The BCI of the females was affected by the reproductive cycle and was highest in August, when parturition is close. In contrast, the males were unaffected by the reproductive cycle, and their BCI was highest in October to increase their survival rate during hibernation.
Climatic factors affecting body condition
The climatic variables affecting G. ussuriensis body condition were PC1 and PC3. High mean annual temperature, high winter temperature, and low annual temperature range, which were represented by PC1, were associated with higher BCI (Table 5; Fig. 3). Generally, an increase in the mean annual temperature results in a more active feeding behavior and larger population size of snakes60,61. For example, when the annual mean temperature increased, the percentage of the feeding individuals of Western whip snakes (Hierophis viridiflavus) increased, and the activity and size of horseshoe whip snakes (Hemorrhois hippocrepis) and Montpellier snakes (Malpolon monspessulanus) increased61,62. Ultimately, an increase in the mean annual temperature results not only in shorter hibernation but also a longer active period and more opportunities for feeding; thus, snakes grow more rapidly and reproduce more frequently, causing an increase in their population size60. In addition, the BCI of our G. ussuriensis specimens increased when the temperature increased during hibernation. This finding is consistent with that of a previous study wherein the snakes had higher survival rates when the temperature was higher during hibernation58. Therefore, a higher annual temperature range may positively affect the body condition of G. ussuriensis. However, when the ambient temperature gets too hot, it may cause negative effects on the body condition of snakes by promoting their metabolism, resulting in excessive energy demands63,64. We found that PC3, which reflects high summer temperatures, had an effect on body condition; however, the trend was unclear, and the relationship between PC3 and body condition should be investigated further through long-term monitoring. Meanwhile, the body condition of G. ussuriensis was unaffected by PC2, which reflects precipitation-related variables. Such a finding is attributed to the greater influence of altitude and ambient temperature than water sources on maintaining the body condition of G. ussuriensis15,17,26, although the results of GLMM did not show significance (Table 5). Moreover, researchers who investigated the effects of the surrounding environment on snakes reported that precipitation-related variables had lesser effect on the range, activity, or population size of snakes than other geographic variables15,61,65. In summary, temperature was the main climatic variable that affected the body condition of our G. ussuriensis specimens; their BCI increased as the mean annual temperature and winter temperature increased.
Intraspecific niche overlap
Altitude was found to be the geographic variable with significant effects on the health of G. ussuriensis. Among the environmental variables, altitude had the greatest effect on the range of three Viperidae species living in Korea, and interspecific ecological niches are delimited by the preferred altitude range of each species15,17,37. For example, G. ussuriensis mainly inhabits low-altitude mountainous forests below 500 m, G. blomhoffii usually inhabits low-altitude rivers and grasslands below 300 m, and G. saxatilis typically inhabits high-altitude mountainous forests above 400 m27,47. Due to differences in their preferred environments, an interspecific niche separation by altitude (PI: 0.27) and habitat (PI: 0.26) was determined between G. ussuriensis and G. saxatilis17. On the other hand, since the ecological habitats of the G. ussuriensis specimens examined in this study were similar among different sexes and ages, there was a very high degree of intraspecific niche overlap by altitude (PI: 0.97) and habitat (PI: 0.99). Nevertheless, the niche overlap, defined by geographic and climatic variables selected by sex and age, between the neonates and adults or between the neonates and juveniles was less than that among adults or between the adults and juveniles. This result is attributed to the observation that the major food sources and habitat types differed by age5,18,22. For example, a study on food source utilization by age in Terciopelo pit vipers (B. asper) revealed that the neonates prefer amphibians and centipedes, while the adults and juveniles prefer mammals66. Meanwhile, turtle-headed sea snakes (Emydocephalus annulatus) show differences in habitat with age: the neonates favor shallow areas with ample rubble, while the adults favor deeper areas with more sand and coral22. This result is attributed to the effect of snake size on the type and size of utilizable food source and their habitat preference; hence, these differences reduce intraspecific competition67. In summary, G. ussuriensis had a higher degree of intraspecific niche overlap depending on altitude, habitat, and climatic factors; the niche overlap among the neonates was less that than among the adults and juveniles.
Conclusion
The analysis of the body size of G. ussuriensis specimens revealed that they have thinner bodies than other Viperidae species, which allows them to move actively and hunt diverse food sources. Unlike typical Viperidae species, the SSD index of G. ussuriensis was positive, meaning that that females are larger than the males. The difference between the body size of males and females was small. Male-to-male combat behavior, which is related to size, has not yet been observed among G. ussuriensis males; thus, further behavioral studies are required. Furthermore, phylogenetic, morphological (e.g., scale count, head length, etc.), and genetic research should be conducted on the Jeju-do population, which had a negative SSD index. Changes in body condition appropriately reflected the life cycle of G. ussuriensis in a sex-specific manner, including reproduction, hibernation, and feeding. Among the climatic variables affecting body condition, the annual mean temperature and winter temperature were positively correlated with body condition because G. ussuriensis requires an appropriate temperature to maintain its high survival rate while hibernating in harsh environments. Although we excluded pregnant females that were close to parturition from the analysis of the environmental variables affecting body condition, we could not completely exclude the effects of pregnancy on body condition because G. ussuriensis females do not reproduce annually, and we could not perfectly detect yolk formation in the early stage of the reproductive cycle in May. There was an extremely high degree of niche overlap between the sexes and development stages of G. ussuriensis. The preferred food resources and habitats of the neonates differ from those of the other age groups; hence, the neonates had less niche overlap than the adults and juveniles The habitats and utilization of food resources by G. ussuriensis of different development stages should be investigated in more detail so that the causes of niche overlap and the mechanisms involved in intraspecific competition will be revealed.
Methods
Survey area
The survey area was the Korean peninsula. Its altitude ranges from 0 to 1950 m: it is high in the eastern region due to the Taebaek Mountain Range; however, it is low in the western region, where paddies, wetlands, and other flat lands are abundant. The land area consists of 65% forest land and 20% agricultural land68. The southern regions include numerous islands of different areas, including Jeju-do (Fig. 5). The mean annual temperature is 13.3 °C, and the annual precipitation is 1244.5 mm. The peninsula has continental climate, with four distinct seasons; it is cold and dry in winter and hot and humid in summer69.
Collection and measurement
The survey was conducted from 2018 to 2022, particularly from April to October. We traveled by foot around valleys, rivers, and agricultural land in the mountainous regions, where G. ussuriensis is commonly found17,26, across the entire Korean peninsula and randomly selected 123 locations for sampling. We collected snake specimens during the day (09:00–18:00) and at night (20:00–01:00). The snakes were captured by hand and through the use of snake tongs or snake hooks. GPS data were used to record the coordinates and date of capture. The SVL, weight, and sex of the specimens were measured using a flexible ruler (to 0.1 cm), spring or balance (to 0.01 g), and ball-tip probe, respectively47. After the measurements, all specimens were released to the same locations where they were caught. To minimize error and improve accuracy, all measurements were taken by three experts who have been studying reptiles for over 20 years (MS, JH, and SC). The capture and measurement of the body size and condition of G. ussuriensis were performed after we had received approval from the Institutional Animal Care and Use Committee of the National Institute of Biological Resources (No. NIBR IACUC 20220001). Sample collection, measurement, and analysis were performed in accordance to the guidelines and regulations of NIBR IACUC.
Based on SVL, the development stages of G. ussuriensis are classified into neonate, juvenile, and adult8. Neonate (< 1 year old), juvenile, and adult specimens are less than 25 cm long, less than 40 cm long, and 40 cm or longer, respectively54. The weight of offspring has significant effects on the body condition of pregnant females. We identified the pregnant females by feeling the developing offspring in their abdomen and recognizing the signs of impending parturition (e.g., late gestation: 30–37 days). The female specimens whose reproductive stage (e.g., yolk stage, early gestation: 19–27 days) was uncertain were not included among the pregnant females6,51,55.
Body size and body condition index
To determine the body size of G. ussuriensis, the mass-to-SVL ratio was calculated using Eq. (1):
where mass (M, g) is plotted against SVL (cm).
Sexual size dimorphism (SSD) was calculated using Eq. (2)70:
Generally, this index is negative when males are larger and positive when females are larger; a zero score indicates symmetry.
Typically, the BCI of snakes is measured from the residuals of the ordinary least squares regression of M on SVL10,11. In this study, we calculated the “scaled mass index” (({widehat{M}}_{i})) based on the reduced major axis (RMA), which can best predict the changes in fat and protein stores of animals and is less affected by errors2,12,71. ({M}_{i}) is the weight of each individual, while ({SVL}_{i}) and ({SVL}_{O}) pertain to the SVL of an individual and the mean SVL, respectively. ({b}_{RMA}) is the scaling exponent estimated by the RMA regression of M on SVL. BCI allows all individuals to be normalized to the same body size, and is not affected by growth, sex, or age. BCI was calculated using Eq. (3)71:
The analysis of variance (ANOVA) of the BCI by year and sex in July and August, when G. ussuriensis were abundant and the sample size was large, revealed no statistically significant differences (p > 0.5). We assumed that the data collected over several years to compare the monthly BCI were collected quantitatively. The logarithm (log) of the BCI was used to present the final results2,72. Factorial ANOVA was employed to investigate the body size and condition of G. ussuriensis. The SVL and mass-to-SVL ratio were utilized to compare the body sizes of males and females in each development stage (adult, juvenile), and log BCI was applied to compare the body conditions of males and females at different time points (monthly). Fisher’s LSD test was employed to evaluate the differences between the mean values and identify significant variables. Descriptive results are reported as mean ± standard error.
Geographical environment
By using ArcGIS (v. 10.3; Esri, California, USA), we projected the locations where G. ussuriensis was observed onto geographical and climatic environment maps15,37. For the altitude map, we utilized a digital elevation model (cell size: 10 m) provided by the National Geographic Information Institute. For the habitat map, we employed the Global Cover (2021 v. 2.0) map (cell size: 10 m) provided by the European Space Agency. For climate data, we used 19 modern (average 1960–1990) climate maps (cell size: 1 km) obtained from Worldclim v. 1.4 (Table 1). Since climate variables are strongly correlated, we performed principal component analysis (PCA) in this study; in accordance with the Kaiser rule, we included three principal components (PCs) with eigenvalues > 1 (Table 4).
Environmental variables affecting body condition
BCI is often used to investigate the life cycle (reproduction, hibernation, active period, etc.) and other physiological, ecological, and behavioral characteristics of a species by sex, without the need to differentiate pregnant females2,3,71. However, because pregnant females have higher BCI than non-pregnant females and males, we excluded pregnant females (n = 32) from the analysis of environmental factors affecting body condition to minimize error. The main environmental factors affecting the body condition of G. ussuriensis were analyzed using a generalized linear mixed model (GLMM) with a Poisson distribution. The dependent variable was log BCI, while the independent variables were habitat, altitude, and the three PCs representing climate (Table 6). The survey year and month used as a random effect, and ANOVA was employed to calculate the likelihood ratio test for the fitted model. For the statistically significant geographic and climatic variables, scatter plots were used to visualize the results and examine the relationships of the environmental factors with the body condition of the specimens.
Intraspecific niche overlap
To analyze the extent of intraspecific niche overlap for G. ussuriensis, the specimens were divided into five categories based on sex and development stage (female adults, female juveniles, male adults, male juveniles, and neonates). The Pinaka index (PI), which reflects niche overlap, was calculated in EcoSim using the geographic and climatic variables in the regions where the specimens were observed73. A PI of 1.0 indicates complete niche overlap, while a PI of 0 indicates complete niche separation. The statistical significance of the niche overlap was calculated using a random algorithm in EcoSim73. To investigate the extent of niche overlap relative to sex and development stage, which could be explained by altitude and PC1–PC3 from PCA, we generated a kernel probability density plot. All statistical analyses were performed using R 4.1.374.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
-
Pearson, D., Shine, R. & Williams, A. Geographic variation in sexual size dimorphism within a single snake species (Morelia spilota, Pythonidae). Oecologia 131, 418–426 (2002).
-
Sivan, J., Kam, M., Hadad, S., Degen, A. A. & Rosenstrauch, A. Body size and seasonal body condition in two small coexisting desert snake species, the Saharan sand viper (Cerastes vipera) and the crowned leafnose (Lytorhynchus diadema). J. Arid Environ. 114, 8–13 (2015).
-
Frank, K. & Dudas, G. Body size and seasonal condition of Caspian whip snakes, Dolichophis caspius (Gmelin, 1789), in southwestern Hungary. Herpetozoa 30, 131–138 (2018).
-
Glaudas, X., Rice, S. E., Clark, R. W. & Alexander, G. J. Male energy reserves, mate-searching activities, and reproductive success: alternative resource use strategies in a presumed capital breeder. Oecologia 194, 415–425 (2020).
-
Shine, R. Why do larger snakes eat larger prey items?. Funct. Ecol. 5, 493–502 (1991).
-
Reyes, M., Zandberg, K., Desmawati, I. & de Bellard, M. E. Emergence and migration of trunk neural crest cells in a snake, the California kingsnake (Lampropeltis getula californiae). BMC Dev. Biol. 10, 52 (2010).
-
Lind, C. M., Moore, I. T., Vernasco, B. J. & Farrell, T. M. Seasonal testosterone and corticosterone patterns in relation to body condition and reproduction in a subtropical pitviper, Sistrurus miliarius. Gen. Comp. Endocrinol. 267, 51–58 (2018).
-
Shine, R. & Charnov, E. L. Patterns of survival, growth, and maturation in snakes and lizards. Am. Nat. 139, 1257–1269 (1992).
-
Shine, R. Sexual size dimorphism in snakes revisited. Copeia 1994, 326–346 (1994).
-
McArdle, B. H. The structural relationship: Regression in biology. Can. J. Zool. 66, 2329–2339 (1988).
-
Moore, I. T., Lerner, J. P., Lerner, D. T. & Mason, R. T. Relationships between annual cycles of testosterone, corticosterone, and body condition in male red-spotted garter snakes, Thamnophis sirtalis concinnus. Physiol. Biochem. Zool. 73, 307–312 (2000).
-
Peig, J. & Green, A. J. The paradigm of body condition: A critical reappraisal of current methods based on mass and length. Funct. Ecol. 24, 1323–1332 (2010).
-
Nafus, M. G., Yackel Adams, A. A. Y., Boback, S. M., Siers, S. R. & Reed, R. N. Behavior, size, and body condition predict susceptibility to management and reflect post-treatment frequency shifts in an invasive snake. Glob. Ecol. Conserv. 21, e00834 (2020).
-
Luiselli, L. Resource partitioning and interspecific competition in snakes: The search for general geographical and guild patterns. Oikos 114, 193–211 (2006).
-
Do, M. S., Lee, J. W., Jang, H. J., Kim, D. I. & Yoo, J. C. Interspecific competition and spatial ecology of three species of vipers in Korea: An application of ecological niche-based models and GIS. Korean J. Environ. Ecol. 30, 173–184 (2016).
-
Pianka, E. R. The structure of lizard communities. Annu. Rev. Ecol. Syst. 4, 53–74 (1973).
-
Do, M. S. & Nam, K. B. Distribution patterns and ecological niches of the red-tongued pit viper (Gloydius ussuriensis) and the Central Asian pit viper (Gloydius intermedius) in Cheonmasan Mountain, South Korea. Russ. J. Herpetol. 28, 348–354 (2021).
-
Shine, R., Harlow, P. S., Keogh, J. S. & Boeadi, A. The influence of sex and body size on food habits of a giant tropical snake, Python reticulatus. Funct. Ecol. 12, 248–258 (1998).
-
Loebens, L., Hendges, C. D., Almeida-Santos, S. M. & Cechin, S. Z. Morphological variation and sexual dimorphism in two sympatric dipsadine snakes from Southern Brazil. Zool. Anz. 280, 42–51 (2019).
-
Ricklefs, R. E. The Economy of Nature (W. H. Freeman and Co., 1998).
-
Rivas, J. A. & Burghardt, G. M. Understanding sexual size dimorphism in snakes: Wearing the snake’s shoes. Anim. Behav. 62, F1–F6 (2001).
-
Shine, R., Shine, T. & Shine, B. Intraspecific habitat partitioning by the sea snake Emydocephalus annulatus (Serpentes, Hydrophiidae): The effects of sex, body size, and colour pattern. Biol. J. Linn. Soc. 80, 1–10 (2003).
-
Gloyd, H. K. & Conant, R. Snakes of the Agkistrodon Complex, A Monographic Review. 28–29 (Society for the Study of Amphibians and Reptiles, 1990).
-
Zhao, E. China Red Data Book of Endangered Animals (Amphibia and Reptilia) (Science Press, 1998).
-
Lee, J. H., Jang, H. J. & Suh, J. H. Ecological Guide Book of Herpetofauna in Korea. 218–231 (National Institute of Environmental Research, 2011).
-
Do, M. S. & Yoo, J. C. Distribution pattern according to altitude and habitat type of the red-tongue viper snake (Gloydius ussuriensis) in the Cheon-ma mountain. J. Wetlands Res. 16, 19–204 (2014).
-
Do, M. S. et al. The establishment of ecological conservation for herpetofauna species in hotspot areas of South Korea. Sci. Rep. 12, 14839 (2022).
-
Oh, H. S. & Banjade, M. Ecology of red-tongue viper (Gloydius ussuriensis) in Jeju Island, South Korea. In Snake Venom and Ecology (eds. Mohammad, M. S., Umar, S., Tijjani, R. B. & Tijjani, S. I.). 1st Ed. 63–76 (IntechOpen, 2021).
-
Ji, D., Weng, S. & Liu, M. Classification of Agkistrodon halys in Northeast China. In Current Herpetology in East Asia (eds. Matsui, M., Hikida, T. & Goris, R. C.). 185–192 (Herpetological Society of Japan, 1989).
-
Do, M. S. & Lee, S. C. First record of a tapeworm (genus Spirometra) in the oral cavity of a viper Gloydius ussuriensis in South Korea. Herpetol. Notes 16, 373–376 (2023).
-
Paik, N. K., Kim, Y. J. & Yang, S. Y. Biochemical variation and systematic status of the genus Agkistrodon (Crotalidae) in Korea. Korean J. Zool. 22, 153–164 (1979).
-
Lee, Y. S. et al. Phylogenetic relationships between three Korean pit viper Gloydius (Serpentes: Crotalinae) species using mitochondrial DNA genes. Genes Genomics 44, 517–526 (2022).
-
Kim, B. S. & Oh, H. S. Sexual size dimorphism in the red-tongued viper snake (Gloydius ussuriensis) of population. Korean J. Environ. Ecol. 28, 542–549 (2014).
-
Mori, A., Ji, D., Moriguchi, H. & Hasegawa, M. Food habits of snakes in East Asia: A biogeographical approach to resource partitioning. In Current Herpetology in East Asia (eds. Matsui, M., Hikida, T. & Goris, R. C.). 433–436 (Herpetological Society of Japan, 1989).
-
Kim, B. S. & Oh, H. S. Foods use of the red-tongued viper snake (Gloydius ussuriensis). Korean J. Environ. Ecol. 28, 657–663 (2014).
-
Debono, J., Bos, M. H. A., Do, M. S. & Fry, B. G. Clinical implications of coagulotoxic variations in mamushi (Viperidae: Gloydius) snake venoms. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 225, 108567 (2019).
-
Do, M. S., Choi, S., Jang, H. J. & Suh, J. H. Predicting the distribution of three Korean pit viper species (Gloydius brevicaudus, G. ussuriensis, and G. intermedius) under climate change. Russ. J. Herpetol. 29, 262–274 (2022).
-
Feldman, A. & Meiri, S. Length–mass allometry in snakes. Biol. J. Linn. Soc. Lond 108, 161–172 (2013).
-
Fitch, H. S. Sexual size differences in reptiles. Ibid 70, 1–72 (1981).
-
Sazima, I. Natural history of the jararaca pit viper, Bothrops jararaca, in Southeastern Brazil. In Biology of the Pit Vipers (eds. Campbell, J. A. & Brodie, E. D.). 199–216 (Selva, 1992).
-
Greene, H. W. The ecological and behavioral context for pit viper evolution. In Biology of the Pit Vipers (eds. Campbell, J. A. & Brodie, E. D.). 107–117 (Selva, 1992).
-
Greene, H. W. Snakes: The Evolution of Mystery in Nature (University of California Press, 1997).
-
Martins, M., Marques, O. & Sazima, I. Ecological and phylogenetic correlates of feeding habits in neotropical pit vipers of the genus Bothrops. Biol. Vipers 307, 328 (2002).
-
Egler, S. G., Oliveira, M. E. & Martins, M. Bothrops atrox (common lancehead). Foraging behavior and ophiophagy. Herpetol. Rev. 27, 22–23 (1996).
-
Choi, W. et al. Diets of four snake species in South Korean mountain forests. J. Asia Pac. Biodivers. 15, 495–499 (2022).
-
Lee, J. H. & Park, D. Determining home range of Gloydius ussuriensis using radiotelemetry: preliminary results. In The 1st Korean Amphibian and Reptiles Conference Data Book (eds. Lee, S. C., Suh, J. H. & Song, J. Y.). 2–3 (Korean Society of Herpetologists, 2008).
-
Do, M. S., Nam, K. B. & Yoo, J. C. Distribution and movement tendencies of short-tailed viper snakes (Gloydius saxatilis) by altitude. Asian Herpetol. Res. 8, 39–47 (2017).
-
Do, M. S. Habitat use and hiding behavior of Central Asian pit viper (Gloydius intermedius). Korean J. Herpetol. 12, 1–8 (2021).
-
Jo, Y. S., Baccus, J. T. & Koprowski, J. L. Mammals of Korea. 464–540 (National Institute of Biological Resources, 2018).
-
Shine, R. et al. Body size enhances mating success in male garter snakes. Anim. Behav. 59, F4–F11 (2000).
-
Do, M. S., Nam, K. B. & Yoo, J. C. First observation on courtship behavior of short-tailed viper snake, Gloydius saxatilis (Squamata: Viperidae) in Korea. J. Asia Pac. Biodivers. 10, 583–586 (2017).
-
Slip, D. J. & Shine, R. Feeding habits of the diamond python, Morelia s. spilota: Ambush predation by a boid snake. J. Herpetol. 22, 323–330 (1988).
-
Hammond, S. Life history notes: Morelia spilotes variegata (carpet python). Male combat. Herpetol. Rev. 19, 37 (1988).
-
Kim, B. S., Chang, M. H. & Oh, H. S. Growth pattern of red-tongued viper snake (Gloydius ussuriensis) inhabiting Gapado, Jeju Island. J. Environ. Impact Assess. 25, 477–486 (2016).
-
Naulleau, G. & Bonnet, X. Body condition threshold for breeding in a viviparous snake. Oecologia 107, 301–306 (1996).
-
Aubret, F., Bonnet, X., Shine, R. & Lourdais, O. Fat is sexy for females but not males: The influence of body reserves on reproduction in snakes (Vipera aspis). Horm. Behav. 42, 135–147 (2002).
-
Hirth, H. F. Weight changes and mortality of three species of snakes during hibernation. Herpetologica 22, 8–12 (1966).
-
Kissner, K. J. & Weatherhead, P. J. Phenotypic effects on survival of neonatal northern water snakes Nerodia sipedon. J. Anim. Ecol. 74, 259–265 (2005).
-
Gregory, P. T. Reptilian hibernation. In Biology of the Reptilia (eds. Gans, C. & Pough, F. H.). Vol. 12. 53–154 (Academic, 1982).
-
Rugiero, L., Milana, G., Petrozzi, F., Capula, M. & Luiselli, L. Climate-change-related shifts in annual phenology of a temperate snake during the last 20 years. Acta Oecol. 51, 42–48 (2013).
-
Capula, M. et al. Long-term, climate-change-related shifts in feeding frequencies of a Mediterranean snake population. Ecol. Res. 31, 49–55 (2016).
-
Zamora-Camacho, F. J., Pleguezuelos, J. M. & Moreno-Rueda, G. Long- and short-term impact of temperature on snake detection in the wild: Further evidence from the snake Hemorrhois hippocrepis. Acta Herpetol. 5, 143–150 (2010).
-
Lourdais, O., Shine, R., Bonnet, X., Guillon, M. & Naulleau, G. Climate affects embryonic development in a viviparous snake, Vipera aspis. Oikos 104, 551–560 (2004).
-
Dupoué, A., Brischoux, F. & Lourdais, O. Climate and foraging mode explain interspecific variation in snake metabolic rates. Proc. Biol. Sci. 284, 20172108 (2017).
-
Capula, M. et al. Long-term, climate change-related shifts in monthly patterns of roadkilled Mediterranean snakes (Hierophis viridiflavus). Herpetol. J. 24, 97–102 (2014).
-
Loaiza-Lange, A. et al. Feeding ecology of the Terciopelo pit viper snake (Bothrops asper) in Ecuador. PeerJ 11, e14817 (2023).
-
Best, T. L. & Gennaro, A. L. Feeding ecology of the lizard, Uta stansburiana, in southeastern New Mexico. J. Herpetol. 18, 291–301 (1984).
-
NGII (National Geographic Information Institute). Digital Topographic Map. https://www.ngii.go.kr (2023).
-
KMA (Korea Meteorological Administration). National Climate Data Center Weather Database. https://data.kma.go.kr/ (2023).
-
Lovich, J. E. & Gibbons, J. W. A review of techniques for quantifying sexual size dimorphism. Growth Dev. Aging 56, 269–281 (1992).
-
Peig, J. & Green, A. J. New perspectives for estimating body condition from mass/length data: The scaled mass index as an alternative method. Oikos 118, 1883–1891 (2009).
-
Sokal, R. & Rolf, F. Biometry: The Principals and Practice of Statistics in Biology Research (WF Freeman, 1995).
-
Entsminger, G. L. EcoSim Professional: Null Modeling Software for Ecologists, Version 1. http://www.garyentsminger.com/ (Acquired Intelligence Inc., 2014).
-
R Core Team. R: A Language and Environment for Statistical Computing. http://www.R-project.org/ (R Foundation for Statistical Computing, 2022).
Funding
This work was supported by a grant from the National Institute of Biological Resources, funded by the Ministry of Environment, Republic of Korea [NIBR No. 202206102].
Author information
Authors and Affiliations
Contributions
M.S.D. and H.K.N. contributed to the study conception and design. Data collection was performed by M.S.D., J.H.J., and S.C.L. Material preparation and analysis were performed by M.S.D., S.J.S., G.C., and H.K.N. The first draft of the manuscript was written by M.S.D. and H.K.N. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Do, M.S., Son, SJ., Jung, JH. et al. Effects of environmental factors and intraspecific niche overlap on the body and ecological characteristics of red-tongued pit vipers (Gloydius ussuriensis).
Sci Rep 13, 21310 (2023). https://doi.org/10.1038/s41598-023-48707-z
-
Received: 08 July 2023
-
Accepted: 29 November 2023
-
Published: 03 December 2023
-
DOI: https://doi.org/10.1038/s41598-023-48707-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.