The US Food and Drug Administration (FDA) has released its proposed rule on front-of-package (FOP) nutrition labeling.
Announced on January 14, 2025, the proposed rule would add a requirement to include an informational box that highlights information on saturated fat, sodium, and added sugars – described as “nutrients to limit” – on the principal display panel of most food labels, or on the labeling of foods sold to consumers from bulk containers. It also seeks to update certain nutrient content claim regulations to better align with current science and avoid within-label inconsistencies related to the three nutrients at issue. FDA has been considering FOP nutrition labeling since 2007.
This proposal is part of a broader White House National Strategy on Hunger, Nutrition, and Health to reduce the burdens of diet-related chronic diseases. If finalized, it would underscore FDA’s goal by providing accessible, at-a-glance nutrition information, helping consumers efficiently identify the role of a given food in a healthy diet and allowing them to compare nutrition information related to the three referenced nutrients across foods.
Overview of FDA’s proposed requirements
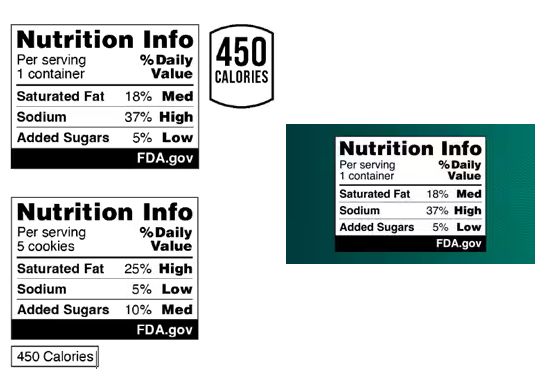
FDA’s proposed FOP nutrition label, referred to as the “Nutrition Info” box, would list the Daily Value (DV) percentage of saturated fat, sodium, and added sugars and would also interpret those percentages to communicate their relative significance in the context of a total daily diet. The interpretations would be listed in bold, to the right of the percent DV, and provide a description using one of the following: “Low,” “Med,” or “High.” The proposed Nutrition Info box aims to help consumers understand the correlation between percentage DV and contribution of the three specified nutrients to their daily diet.
The Nutrition Info box would be required for most foods marketed to people ages four or older that bear a Nutrition Facts label. FDA tentatively concluded that a failure to display the Nutrition Info would fail to provide consumers with interpretive nutrition information. In accordance with existing regulations, manufacturers could voluntarily include a calorie statement on the front of the food package.[1]
Additionally, this proposed rule, if finalized, would also amend the low sodium and low saturated fat nutrient content claim regulations to align with current nutrition science and avoid within-label inconsistencies. In short, these nutrient content claims for sodium and saturated fat cannot be made unless they display “low” in accordance with the FOP requirements.
FDA has established some exemptions and modified requirements for some food categories. For example, FDA currently proposes an exemption from the Nutrition Info box for raw fruits and vegetables and small packages with a total surface area of fewer than 12 square inches. There are also modified requirements for foods in packages with a total surface area of 40 or fewer square inches. However, FDA tentatively concluded that there would not be an exemption from the requirement to display the Nutrition Info box for foods that contain insignificant amounts of saturated fat, sodium, and added sugars from the requirement. Additionally, FDA did not propose to exempt electrolyte drinks, glucose products, and nutrition shakes from the requirement to display a Nutrition Info box.
The interpretive descriptions
In proposing the nutrient level descriptions (“Low,” “Med,” or “High”), FDA considered the regulatory history related to the establishment of the percent DV, its consumer education activities designed to help consumers understand percent DV in the context of the total daily diet, nutrition education initiatives of other groups, and existing FDA definition for nutrient content claims, including definitions already established for “low” and “high” claims. To that end, FDA proposed the following interpretive description range to the Nutrition Info box:
- “Low”: 5-percent DV or less
- “Med”: 6- to 19-percent DV
- “High”: 20-percent DV or more
FDA notes in its proposed rule that required information appearing in the Nutrition Info box is not a nutrient content claim and thus is not subject to the requirements of nutrient content claims under Section 403(r) of the Food, Drug, and Cosmetic Act (FD&C Act).
Nutrition Info box content requirements
The proposed rule would require a heading entitled “Nutrition Info” that would be similar in appearance to the “Nutrition Facts” title of the Nutrition Facts label.[2] FDA proposed using the subheading “Per serving” akin to the Nutrition Facts label, to help consumers understand that the Nutrition Info box provides information about one serving of food. FDA also proposed including a statement of the serving size along the “Per serving” subheading, expressed in household measures, but would not require inclusion of the serving’s gram amount in the Nutrition Info box.
Additionally, under this proposed rule, FDA would require the inclusion of the subheading “% Daily Value” in the box above the declaration of the quantitative percent DV and the interpretative description. Resulting from the significance in building healthy dietary patterns, scientific research, and current food labeling, the nutrients in the proposed box would be saturated fat, sodium, and added sugars.
The following visual illustrates the requirements for a Nutrition Info box, including an adjacent optional calorie statement. It also provides examples of how the optional calorie statement might be presented.

Nutrition Info box placement and format
If finalized, the Nutrition Info box would appear on the upper third of the principal display panel. It would require an easy-to-read font and follow the guidelines already established for information required by FDA. According to the established guidelines, the box must be prominent, conspicuous, and in such terms as to render it likely to be read and understood by the ordinary individual under customary conditions of purchase and use.
FDA has also proposed specific requirements regarding the type size, color, and format of the Nutrition Info box. These include a requirement to bear a “FDA.gov” statement section prominently in the box.
Proposed changes to nutrient content claims
In order to prevent within-label inconsistencies, FDA proposes to amend certain nutrient content claims. These include:
- Lowering the limit of sodium allowed in a product bearing a “low sodium” nutrient content claim from 140 mg or less per RACC or 100 g to 115 mg or less per RACC or 100 g.[3]
- Adding requirements to existing regulations to specify that a food subject to this rule must bear “Low” in accordance with the proposed levels in the Nutrition Info box for sodium and saturated fat in the Nutrition Info box in order to qualify for to bear the related “Low” in the nutrient content claim.
Comment period
Interested stakeholders have until May 16, 2025 to submit comments on this proposed rule.
Proposed compliance date
The proposed rule establishes a compliance date of three years after the final rule’s effective date for businesses with $10 million or more in annual food sales, and a compliance date of four years after the final rule’s effective date for businesses with less than $10 million in annual food sales.
Preemption
Section 403A of the FD&C Act is an express preemption provision. If this proposed rule is finalized, the rule would create requirements that fall within the scope of section 403A(a) of the FD&C Act to the extent that these provisions are consistent with section 403(q) of the FD&C Act.
Key takeaways of the FOP proposal
While the industry has largely been working with FDA to provide more accessible nutrition information on a voluntary basis (eg, “Facts up Front” icons), this proposed requirement may pose challenges for industry. For example, in addition to the cost of compliance, companies may need to eliminate other information and graphics on labels in order to make room for the Nutrition Info box.
It also remains uncertain how the Nutrition Info box would apply to main-dish and meal products. While other FDA rules address the difference among different types of products, this proposed rule remains silent on how FOP requirements would apply in those instances.
Given these concerns, the proposed rule will likely yield numerous comments that may substantially shape any final rule. There will also likely be ample opportunity to engage with new leadership at the Agency to discuss industry concerns and challenges.
Finally, given the change of administration, it is unclear how the finalization of this rule will be prioritized.
[1] See Examples of FDA Proposed Nutrition Info Boxes for additional graphics of the proposed Nutrition Info boxes, including an example of listing calories near the box.
[2] See 27 CFR § 101.9(d) for Nutrition Label formatting requirements.
[3] 21 CFR § 101.61(b)(4)-(5) (listing current “low sodium” nutrient content claim thresholds).
[View source.]