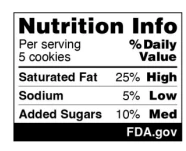
On 14 January 2024, the US Food and Drug Administration (FDA) issued a proposed rule to be published on 16 January, which, if finalized, will require a front-of-package (FOP) nutrition label on most packaged foods. This FOP nutrition label consists of a “Nutrition Info box,” detailing the relative Percent Daily Values (% DV) of saturated fat, sodium, and added sugars (undesirable nutrients) in a serving of the packaged food. The Nutrition Info box for most foods will: (1) include a description of the serving size; (2) include the % DV of the undesirable nutrients; (3) include a characterization of such values as “Low,” “Med,” and “High;” and (4) appear in at least eight-point font or be no smaller than the size of the required net quantity of contents declaration specified in 21 C.F.R. §§ 101.7(h) and (i). The Nutrition Info box will have to be prominently displayed in the upper-right corner of the Principal Display Panel.
The proposed rule would require that most packaged foods for children aged four and older and the general population bear a Nutrition Info box, a sample image of which is shown below:

Image Source: US Food and Drug Administration Website
Levels 5% or less of the % DV of saturated fat, sodium, and added sugars would be considered “Low,” while levels 6% to 19% of the % DV are “Med.” Levels of 20% or more of the % DV are considered “High.”
FDA noted that these numbers are generally consistent with the levels in the “low” (21 C.F.R. § 101.62(b)(2)) and “high” (21 C.F.R. § 101.54(b)) nutrient content claims. However, it is relevant to note that these terms have historically been associated with marketing claims of favorable nutrients (e.g., high in dietary fiber and low in sodium). This proposed rule marks the first time FDA is regulating the mandatory use of the “high” term for undesirable nutrients.
FDA tested with consumers Nutrition Info boxes that used red for High, yellow for Med, and green for Low to designate which nutrients to limit and consume enough of. However, FDA ultimately declined to include colors as study participants found the scheme confusing.
FDA has invited comments on several facets of this proposed rule. Notably, FDA has invited comments on how these requirements are impacted by the nutritional needs of subpopulations, such as children aged one to three years, and the need for or value of interpretive nutrition information for these subpopulations. FDA also invited comment on the inclusion of a mandatory or voluntary quantitative statement of calories in the Nutrition Info box and ways to interpret quantitative calorie information. Additionally, FDA is seeking feedback on use of the “Low” categorization for products that declare a 0% DV for any of these three nutrients, and whether a fourth categorization, such as “Zero” or “Free,” would better indicate that the product is not simply “Low” for that nutrient but contributes 0% to the % DV for that nutrient.
In addition to creating a Nutrition Info box regulation under 21 C.F.R. § 101.6, the proposed rule would revise the requirements for “low sodium” under 21 C.F.R. § 101.61 and “low saturated fat” nutrient content claims under 21 C.F.R. § 101.62. Notably, the permissible limit for “low sodium” claims would be 115 mg, as compared to the current 140 mg level.
FDA will accept comments on the proposed rule until 16 May 2025, at regulations.gov under docket number FDA-2024-N-2910. If and when the proposed rule is finalized, most businesses will have three years after the effective date to comply; small businesses with less than US$10 million in annual food sales will have four years after the effective date to comply.