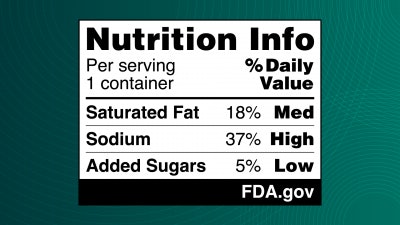
The Food and Drug Administration (FDA) has proposed front-of-package nutrition labels intended to assist consumers in making healthier food choices. This initiative, announced in the closing days of the Biden administration, seeks to provide clear information on the levels of sodium, added sugars, and saturated fats in packaged foods and beverages. The proposed labels would show whether these nutrients are present in low, medium, or high levels, along with their percentage of the daily value they represent.
Packaging World columnist and resident legal expert specializing in food and drug law and packaging law Eric Greenberg is unsure whether the proposed labeling will affect consumers’ food selections.
“This proposal ends a years-long struggle to come up with an effective, abbreviated summary about a food’s character,” Greenberg says. “If it becomes effective, it will be interesting to see if it changes consumer behavior.”
Research has consistently shown that high consumption of sodium, sugar, and saturated fats is linked to chronic diseases like heart disease, cancer, and diabetes, which significantly contribute to disability and healthcare costs in the United States. According to Rebecca Buckner, associate deputy director for human food policy at the FDA’s Human Foods Program, most Americans, despite dietary recommendations, surpass the advised limits for these nutrients.
The proposed labels aim to supplement the existing nutrition facts labels on the back of food packages, providing a more accessible way for consumers to compare the healthfulness of various products. For example, when selecting between two yogurts, the new label would enable consumers to quickly determine which option has less added sugar. This raises a question for Greenberg about whether all consumer packaged goods companies will consider the new labels comprehensive enough to present a fair representation of a product’s contents.
“Simplicity can be good, but not when it’s overdone,” he says. “I expect some food industry advocates will raise concerns that this new proposal oversimplifies the way the Nutrition Info makes some food look ‘bad’ when that isn’t really a fair assessment.”
While the proposal has received support from health advocacy groups like the Center for Science in the Public Interest, which views it as a means to empower consumers, it has also faced criticism from industry representatives. The Consumer Brands Association has raised concerns about the FDA’s methods and the absence of industry input in formulating the proposal. The association points out that existing voluntary labeling programs, such as the Facts Up Front label, are already effective in helping guide consumer choices.
The FDA’s proposal is currently open for public comment until May 16. After collecting comments, the agency may revise or finalize the rule. If implemented, large food manufacturers would have three years to comply, while smaller manufacturers would have an additional year. The proposed labels could potentially lead to reformulations in food products as manufacturers respond to consumer demand for healthier options.
Keeping these timetables in mind, Greenberg encourages food companies to review the proposal thoroughly at their earliest convenience and start readying for potential changes as soon as possible.