Abstract
Efforts to make food systems more sustainable have emphasized reducing adverse environmental impacts of agriculture. In contrast, chemical and biological processes that could produce food without agriculture have received comparatively little attention or resources. Although there is a possibility that someday a wide array of attractive foods could be produced chemosynthetically, here we show that dietary fats could be synthesized with <0.8 g CO2-eq kcal−1, which is much less than the >1.5 g CO2-eq kcal−1 now emitted to produce palm oil in Brazil or Indonesia. Although scaling up such synthesis could disrupt agricultural economies and depend on consumer acceptance, the enormous potential reductions in greenhouse gas emissions as well as in land and water use represent a realistic possibility for mitigating the environmental footprint of agriculture over the coming decade.
Main
The prodigious quantities of food produced by global agriculture entail correspondingly vast areas of land unavailable to natural ecosystems1,2,3, water resources used and polluted4,5, and greenhouse gases (GHGs) emitted to the atmosphere3,6. Efforts to reduce such impacts despite the world’s growing and ever-richer population have focused on limiting demand for the most resource- and pollution-intensive foods7, decreasing the inputs to (and thereby impacts of) agricultural production8 and using produced food more efficiently9,10. Here we highlight another possibility: producing food without agriculture.
An increasing number of academic studies and for-profit ventures have recently demonstrated that edible molecules can be synthesized via chemical and biological processes without need of agricultural feedstocks. Whereas plant-, cell- or fungi-based proteins and meat substitutes made from processed agricultural commodities are increasingly available, synthetically produced food may contain carbon from fossil fuels, waste or the atmosphere—that is, feedstocks that are not the product of agricultural photosynthesis. Futuristic as this may sound, the idea of such non-agricultural food is not novel. Amid the food shortages of World War II, German chemists successfully synthesized a coal-based margarine that was consumed by German citizens and troops11,12. After the war, a German company began synthesizing the amino acid methionine in industrial quantities, initially for treating malnutrition and later as an additive to animal feed13. In the 1960s and 1970s, US chemist Archibald McPherson proposed synthetic food in response to neo-Malthusian population projections, including in a letter published in the journal Nature14,15. Yet the prospects for producing food in this way raises many questions, prominent among them: what kind of food might feasibly be synthesized and with what advantages over agricultural products?
Here we introduce the range of options for producing food without agricultural inputs and then analyse with greater specificity the potential for synthesizing dietary fats. Details of our analytic approach are given in Methods. In summary, we estimate and compare lifecycle energy use by, and GHG emissions from, fats produced by conventional agriculture with molecularly identical fats produced by chemical synthesis, in each case across a range of land-use and energy scenarios. We use estimates of emissions and land use from recent literature, and we convert non-CO2 to CO2-eq GHG using 100-yr global warming potentials, including legacy land-use-change emissions.
Results
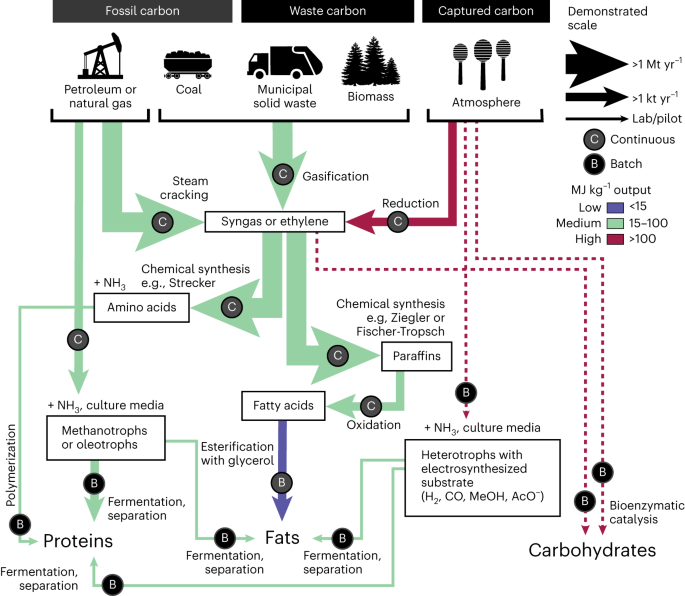
Figure 1 summarizes several potential pathways for synthesizing fats, proteins and carbohydrates from different carbon feedstocks, indicating the current scales of each process by the weight of arrows. Syngas (a mixture of H2 and CO) and ethylene (C2H4) are now produced from fossil fuels and organic wastes at a scale of >1 Mt yr−1 (via gasification and steam cracking, respectively)16. Such syngas and ethylene may then be converted by processes such as Ziegler or Fischer-Tropsch to paraffins, fatty acids and fats17; by processes such as Strecker to amino acids and then proteins18; or by electrochemical catalysis and processes such as the Formose reaction to methanol and then carbohydrates19. Each of these pathways has been successfully demonstrated, although most occur today at relatively small scales (with the important exception of the amino acid methionine, ~1.4 Mt of which was synthesized industrially in 2020 (ref. 13)). Biological pathways to fats, proteins and carbohydrates also exist. Bioenzymatic catalysis has produced carbohydrates from atmospheric carbon20, and all macronutrients have been produced from atmospheric carbon via fermentation of electrosynthetic intermediates, including hydrogen18 and acetate21. Fats and proteins have also been produced from fossil carbon via methanotrophs and oleotrophs22,23,24,25.

Proteins, fats and carbohydrates can be synthesized from a range of carbon feedstocks via multiple chemical and biological pathways (arrows). The weight and colour of the arrows indicate the scale at which the different processes have been demonstrated and the energy required per mass unit output, respectively (see Supplementary Fig. 1 and Supplementary Table 1 for references). Dashed lines indicate where energy requirements remain highly uncertain. Circular labels on each arrow further indicate whether the process is typically continuous (C) or batched (B). NH3 is ammonia, H2 is hydrogen gas, MeOH is methanol and AcO– is acetate. In the context of this study, we exclude agriculturally produced carbon feedstocks.
Although biological and chemical pathways appear intermixed in Fig. 1, chirality is in fact a clear dividing line between them. Chemical pathways can access more economical regimes of temperature, pressure, rate constant and equilibrium constant but can struggle to distinguish between left-handed (l) and right-handed (d) molecular forms. A perfect synthesis of glucose (four chiral centres) with no enantioselectivity would produce an edible compound only 1/16th of the time. By contrast, bioenzymatic techniques offer exquisite mechanisms for installing position-specific functional groups but can operate only in the limited range of process conditions in which life exists—mild temperatures and aqueous environments in which not all feedstocks are soluble. The result is a sharp distinction in Fig. 1: chemical techniques prevail for the synthesis of fats (generally achiral) and the amino acids glycine, which is achiral, and methionine, which is digestible in both the l– and d– forms. Bioenzymatic techniques predominate for the synthesis of all other amino acids (at least one chiral centre) and all carbohydrates (many chiral centres)13.
Among these various pathways, we focus on fats in particular because: (1) they are among the simplest nutrients to synthesize thermochemically (that is, achiral and simply structured, compatible with large-scale soap-making and polymer chemistry techniques), (2) they are the only macronutrient successfully synthesized at scale in the past (although not today26), (3) they serve as gustatorily undifferentiated ‘baseload calories’ in many foods and (4) oilcrops such as soy and palm have an enormous environmental footprint globally (for example, >300 Mha or ~7% of agricultural land and 2.89 Gt CO2-eq or ~20% of annual greenhouse gas emissions from agriculture and land-use change in recent years3). Here we focus on land use and GHG emissions, but we recognize that there are many other factors relevant to the sustainability of food production systems.
Depending on the source and quantity of energy used and associated land-use emissions (for example, N2O from fertilizer or CO2 from clearing land), the GHG emissions per kilocalorie of fats produced by agricultural systems range from <1.0 g CO2-eq kcal−1 (for example, soybeans grown in the United States and Europe) to >3 g CO2-eq kcal−1 (for example, palm oil grown in Africa; Fig. 2a). These regional differences reflect differences in the rates of land-use change, the carbon density of cleared land and farming practices. For land-efficient crops, such as oil palm, patterns of land-use change drive differences in emissions intensity; yields and farming practices largely explain variations in emissions among more land-intensive crops such as soybeans (Fig. 2a). In comparison, we estimate that analogous fats synthesized from natural gas feedstock using energy with the carbon intensity of the current average of US electricity would produce ~0.8 g CO2-eq kcal−1, and with near zero emissions if using carbon captured from the air and relying exclusively on non-emitting sources of energy (Fig. 2b). This is considerably less than the >1.5 g CO2-eq kcal−1 of soy grown in Brazil or palm oil grown in Brazil or Indonesia (Fig. 2a). The only case identified where the emissions intensity of synthetic palm oil approaches the current emissions of oilcrops from sub-Saharan Africa (that is, >3 g CO2-eq kcal−1) is the case in which high-carbon intensity energy (for example, coal) is used to reduce a CO2 feedstock and incorporate the resulting carbon into synthetic fats (that is, the lower right corner of Fig. 2b). The contours in Fig. 2b illustrate an important distinction between synthetic fats produced from feedstocks containing internal energy, such as CH4, and those without, such as CO2: in the latter case (corresponding to the bottom half of Fig. 2b), the emissions intensity of the synthetic fats has a very strong dependence on the emissions intensity of the sourced electricity, while emissions for fossil-derived synthetic fats (corresponding to the top half of Fig. 2b) are dominated by the feedstock source. These trends reflect the fact that the energy required to capture and reduce the CO2 feedstock into hydrocarbons is about eight times greater than the energy needed to convert hydrocarbons into synthetic fats. We also estimate that the land area required per kilocalorie of synthetic fat produced would be an order of magnitude smaller than that of agricultural fats: 15–20 million kcal of agricultural fats per hectare per year3 as compared with >1 billion kcal of synthetic fats per hectare per year (see Supplementary Information 1.3 and Supplementary Table 5).

a,b, Shading and contours show grams of CO2-equivalent GHG emissions per kilocalorie of edible fat produced by conventional agriculture (a) and chemical synthesis (b). Agricultural emissions are shown as the sum of land-use emissions (y axis) and energy-related emissions (x axis), and emissions from synthesis are shown as a function of feedstock emissions intensity (y axis) and energy emissions intensity. Red circles denote specific estimates based on literature and assumed values (as labelled; see Methods). Feedstock emissions include process-related conversion of feedstock to CO2—for example, during extraction of natural gas, gasification of coal and the eventual human respiration of fossil feedstock (see Supplementary Table 4).
To assess potential environmental benefits of synthesizing fats at large scales, Fig. 3 shows the magnitude of GHG emissions and agricultural land area related to current global production of the two largest oilcrops: soy and oil palm. Given large disparities in current GHG emissions and land use per kilocalorie of soy and palm oil, synthetic fats might be targeted to avoid disproportionately large quantities of emissions and land use. For example, half of soy and oil palm calories account for ~3/4 of both land use and land-use emissions related to soy and oil palm agriculture (Fig. 3a,b, respectively). In particular, soybeans grown in the United States, Brazil and Argentina represent 79 Mha (65% of land used worldwide for soy and palm oil), and adding to these the oil palm produced in Indonesia and Malaysia accounts for 1.26 Gt CO2-eq emissions annually (78% of global land-use emissions from soy and palm oil agriculture). Although oil palm has no important co-products except oil27, substantial demand for soy protein (for example, meal for animal feed)28 co-produced with soybean oil might limit replacement of soybean oil, unless demand for soy protein could be reduced in parallel to synthetic oil-driven decreases in demand for soybean oil. For example, decreases in demand for chicken and pork due to dietary shifts29 and/or increases in cost-competitive alternative sources of protein (for example, amino acids fed to cell cultures, direct synthesis by microogranisms)30 could greatly reduce demand for soy protein. However, even if only palm oil could be replaced by synthetic oil, roughly 450 MtCO2-eq emissions per year and 20 Mha of biodiverse tropical land use might have been avoided in recent years (29% and 15% of the total emissions and land areas shown in Fig. 3, respectively).

a,b, Cumulative distributions of CO2-equivalent GHG emissions (a) and land (b) related to soy (red bars) and oil palm (blue bars) agriculture in 2017 (ref. 3), sorted from most-to-least emissions and land-use intensiveness, respectively. In each case, a disproportionately large share of emissions and land use is concentrated in a handful of countries. Environmental benefits of synthetic fats would be greatest if such fats were preferentially substituted for those countries’ agricultural products.
Discussion
Numerous pathways for producing edible macronutrients without agriculture have been demonstrated. Our results show that substantial GHG emissions and land use could be avoided per kilocalorie of dietary fats synthesized chemically, potentially cumulating in climate-relevant quantities of emissions and land use avoided. Such benefits could be extremely and increasingly valuable given rising global demand for sustainable dietary fats31. Interestingly, the climate advantages of synthetic fats do not depend on entirely fossil-free systems; sub-Saharan oilcrops have a GHG intensity greater than synthetic fats produced with coal electricity (Fig. 2). In addition, there may be many similar or related environmental and societal benefits, including reduced water use, decreased air and water pollution, improved food security and food sovereignty, resistance to some global catastrophe scenarios26, less need for low-paying and physically demanding agricultural labour, and vast tracts of land made available for reforestation, with attendant benefits to biodiversity and natural carbon sinks.
However, several limitations and caveats apply to our conclusions. First, our assessment is based upon a number of previously published estimates of the carbon and land intensity of agricultural and industrial processes and products. These estimates are in many cases at the level of countries in recent years and may therefore not capture geographical, process-level and scale-dependent details or projected future changes that could be relevant in quantifying potential environmental benefits. More detailed technoeconomic analysis is needed to prioritize economic and environmental opportunities for synthetic foods.
In addition, there are also substantial barriers to large-scale synthesis of foods for human consumption. Cost is one. Especially while the environmental impacts of agriculture are externalized, prices for high-purity synthetic fats may never be lower than the prices of the cheapest agricultural oils. Large-scale thermochemical processes might produce synthetic fats at prices 0–20% greater than current market prices of soybean and palm oil26. In contrast, with carbohydrates selling for <US$0.10 kg−1 in parts of the world, it is difficult to imagine synthetic processes that could compete economically in the near term. Perhaps more challenging than cost is the barrier of social acceptance. Chemical and biological separation techniques and analytical techniques have advanced to the point that synthetic fats, for example, could be consistently produced with higher levels of purity than those of the processed seed oils humans now consume in great quantities. However, in the public eye, the combination of ‘chemistry’ and ‘food’ have a troubled history of premature rollouts, side effects from non-food compounds marketed to dieters, and overapplication of pesticides and preservatives32. Even in non-food contexts, unanticipated environmental consequences of historical transitions from natural to synthetic processes (for example, from natural to synthetic fibres for clothing) suggest there is good reason to be wary of potential downsides33. For example, it is possible that synthetic food production would entail greater quantities of mined minerals and metals than does conventional agriculture, which itself uses substantial quantities of such materials for fertilizer factories and agricultural machinery. Obstacles of cost, consumer preference and lifecycle sustainability warrant further analysis as commercial technologies for synthesizing foods are demonstrated and begin to scale.
Another potential set of downsides involve the impacts on working people, most of all smallholder farmers in the global South. Agriculture currently employs ~1 billion people worldwide, making up ~27% of the global labour force34. However, this share has declined from 44% in the past three decades, during which time productivity of labour in agriculture has greatly increased due mainly to the mechanization of agricultural processes. Indeed, value added per worker in agriculture has increased from US$1,441 in 1991 to ~US$4,000 (ref. 35) in 2019, as land has been reallocated to larger, more productive farms36. Although these trends belie economic hardships for individuals and families no longer employed in agriculture, it is also clear across all countries and throughout time that a smaller agricultural sector is characteristic of more prosperous economies. As economies grow, structural transformation shifts labourers from agriculture into manufacturing and service sectors, which ultimately results in higher incomes for everyone34,37. Producing food without agriculture will be another step in furthering these existing trends, but as with fossil fuel-workers, proactive policies could help minimize the disruptions for agricultural workers and facilitate a just transition of food systems38,39.
Nevertheless, addressing these concerns could provide us with a valuable new tool for reducing and limiting the enormous environmental footprint of agriculture31. Here we have emphasized climate benefits because land-related GHG emissions are not only large but also often very difficult to avoid. (The technical and socio-economic challenges of synthetic foods seem quite manageable in comparison.) We estimate that synthetic fats would require on the order of 100–800 times less water than agricultural analogues (see Supplementary Information 1.4 and Supplementary Table 6) and could be produced continuously anywhere that carbonaceous feedstocks and low-cost, low-emission energy sources are available. Industrializing food production could thus increase the resilience of food systems by eliminating vulnerabilities to, for example, pests, pathogens, extreme weather and trade relations (although increased interdependence of food and energy systems might increase risks related to failure of energy and communication infrastructure)40. Although we have shown that using fossil carbon feedstocks or fossil-energy inputs to synthesize food might reduce GHG emissions per kcal of food produced relative to current agriculture, synthesizing food sustainably would entail renewable energy and atmospheric carbon (including, perhaps, biogenic carbon in waste streams; Fig. 1). In turn, affording sustainable synthetic foods may depend on innovations to reduce the energy and materials required (that is, cost) to capture carbon from the atmosphere41,42,43.
More broadly, the prospect of synthesized foods invites reflection of humanity’s relationship with nature. The domestication of plants was a watershed in the history of our species. Followed up by the discovery and commercialization of the Haber-Bosch process for fixing nitrogen, the human population has boomed—half of the nitrogen in human bodies is of synthetic origin44. However, we now use the majority of the planet’s habitable land and drinkable water to grow food45, and pour hard-won and energy-intensive nitrogen on the ground where less than 20% is incorporated in crops46. Synthetic food would not only avoid the environmental burdens of agriculture but could begin to reduce our parasitism on plants, reconciling expansive restoration and protection of natural ecosystems47 with human food security.
Methods
Synthetic fat production
Emissions associated with synthetic fat production are calculated using the following contributions: feedstock extraction and delivery (that is, drilling/mining and transport of fossil feedstocks), oxidation of feedstock during both processing and respiration, and energy emissions during processing. The lifecycle emissions are calculated according to equation (1):
where ({eta }_{{rm{feedstock}}}) is the utilization efficiency of the feedstock(left(frac{{rm{{kg}}_{{product}}}}{{rm{{kg}}_{{feedstock}}}}right)) and (rm{{{CI}}_{{energy}}}) is the emissions intensity of the energy source (left(frac{rm{{kg},C{O}_{2e}}}{{rm{MJ}}}right)).
Coal, natural gas and CO2 are selected as representative feedstocks to illustrate trends in the emissions intensity of synthetic fat production. The parameters used in equation (1) for each of the selected feedstocks are shown in Supplementary Table 8; further details associated with the calculation of these parameters are included in the Supplementary Tables 2–4.
Figure 2b was generated by sweeping the emissions intensity of energy (CIenergy in equation (1)) between 0 g CO2-eq MJ−1 (lower bound of renewable energy lifecycle emissions) and 278 g CO2-eq MJ−1 (upper bound of coal energy lifecycle emissions)48 for each of the three scenarios described in Supplementary Table 8, yielding values for emissions intensity along three horizontal cuts. The remaining data in the colormap were filled in by interpolating between these horizontal cuts along the vertical axis. Although CO2 does not have any associated extraction or oxidation feedstock emissions, the production energy from CO2 feedstock included the energy required to capture (9 MJ kg−1 CO2 captured from air49) and reduce CO2 to hydrocarbons at a 3:1 ratio of green H2 to CO2, at an assumed 70% efficiency50 (using 171.4 MJ kg−1 H2).
Agricultural fat production
GHG emissions related to agricultural fat production were estimated as the sum of energy-related emissions and land-use emissions. In the case of land-use emissions, our analysis used country- and product-specific estimates in 2017 from ref. 3, which varied considerably (Supplementary Table 7). More recent product- and country-specific estimates were not available, but the magnitudes of regional land-use change and agricultural emissions have changed little since 2017 (refs. 51,52). Non-CO2 GHGs were converted to CO2-eq using 100-yr global warming potentials (GWPs). Energy-related emissions were derived from the references indicated in Supplementary Table 7, assuming, where necessary, lifecycle carbon intensities as in Supplementary Table 8.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
We used data from relevant literature as cited in our study. All data used in this study are included as Supplementary Information and are also publicly available at https://github.com/proffate/foodwithoutag. Source data are provided with this paper.
Code availability
Data analysis was conducted in MATLAB (v.9.11.0.1809720 (R2021b) Update 1). The code used in this study is included as Supplementary Information and is also publicly available at https://github.com/proffate/foodwithoutag.
References
-
Foley, J. A. et al. Global consequences of land use. Science 309, 570–574 (2005).
Google Scholar
-
Newbold, T. et al. Global effects of land use on local terrestrial biodiversity. Nature 520, 45–50 (2015).
Google Scholar
-
Hong, C. et al. Global and regional drivers of land-use emissions in 1961–2017. Nature 589, 554–561 (2021).
Google Scholar
-
Qin, Y. et al. Flexibility and intensity of global water use. Nat. Sustain. 2, 515–523 (2019).
Google Scholar
-
Evans, A. E., Mateo-Sagasta, J., Qadir, M., Boelee, E. & Ippolito, A. Agricultural water pollution: key knowledge gaps and research needs. Curr. Opin. Environ. Sustain. 36, 20–27 (2019).
Google Scholar
-
Crippa, M. et al. Food systems are responsible for a third of global anthropogenic GHG emissions. Nat. Food 2, 198–209 (2021).
Google Scholar
-
Tilman, D. & Clark, M. Global diets link environmental sustainability and human health. Nature 515, 518–522 (2014).
Google Scholar
-
Garnett, T. et al. Sustainable intensification in agriculture: premises and policies. Science 341, 33–34 (2013).
Google Scholar
-
Cassidy, E. S., West, P. C., Gerber, J. S. & Foley, J. A. Redefining agricultural yields: from tonnes to people nourished per hectare. Environ. Res. Lett. 8, 034015 (2013).
Google Scholar
-
Barrera, E. L. & Hertel, T. Global food waste across the income spectrum: implications for food prices, production and resource use. Food Policy 98, 101874 (2021).
Google Scholar
-
Stranges, A. N. A history of the Fischer-Tropsch synthesis in Germany 1926–45. Stud. Surf. Sci. Catal. 163, 1–27 (2007).
Google Scholar
-
Imhausen, A. Die Fettsäure-Synthese und ihre Bedeutung für die Sicherung der deutschen Fettversorgung. Kolloid-Z. 103, 105–108 (1943).
Google Scholar
-
Drauz, K. et al. Amino acids. Ullmann’s Encycl. Ind. Chem. https://doi.org/10.1002/14356007.a02_057.pub2 (2007).
Google Scholar
-
McPherson, A. T. Chemical and biochemical productioon of food for man and animal. J. Anim. Sci. 25, 575–581 (1966).
Google Scholar
-
McPherson, A. T. Synthetic food. Nature 242, 144–145 (1973).
Google Scholar
-
Annual Energy Outlook (EIA, 2021).
-
Shah, J., Arslan, E., Cirucci, J., O’Brien, J. & Moss, D. Comparison of oleo- vs petro-sourcing of fatty alcohols via cradle-to-gate life cycle assessment. J. Surfactants Deterg. 19, 1333–1351 (2016).
Google Scholar
-
Leger, D. et al. Photovoltaic-driven microbial protein production can use land and sunlight more efficiently than conventional crops. Proc. Natl Acad. Sci. USA 118, e2015025118 (2021).
Google Scholar
-
Soland, N. E., Roh, I., Huynh, W.-S. & Yang, P. Synthesis of carbohydrates from methanol using electrochemical partial oxidation over palladium with the integrated formose reaction. ACS Sustain. Chem. Eng. 11, 12478–12483 (2023).
Google Scholar
-
Cai, T. et al. Cell-free chemoenzymatic starch synthesis from carbon dioxide. Science 373, 1523–1527 (2021).
Google Scholar
-
Hann, E. C. et al. A hybrid inorganic–biological artificial photosynthesis system for energy-efficient food production. Nat. Food 3, 461–471 (2022).
Google Scholar
-
Tawfik, N. I., Khalil, M. A. A.-G. & Abou-Zeid, A. A.-Z. Utilization of petroleum fractions for the production of single-cell protein. Zentralbl. Bakteriol. Naturwiss. 136, 433–448 (1981).
Google Scholar
-
Petersen, L. A. H. Single Cell Protein Production in U-loop Bioreactors: Fundamentals, Modeling and Control. Ph.D. thesis, Technical Univ. Denmark (2019).
-
Groenewald, M. et al. Yarrowia lipolytica: safety assessment of an oleaginous yeast with a great industrial potential. Crit. Rev. Microbiol. 40, 187–206 (2013).
Google Scholar
-
Calysta. Food and Energy Security Through Sustainable Life Sciences http://www.ascension-publishing.com/ABLC-NEXT-2014/Calysta-Shaw.pdf (2014).
-
Martinez, J. B. G., Alvarado, K. A. & Denkenberger, D. C. Synthetic fat from petroleum as a resilient food for global catastrophes: preliminary techno-economic assessment and technology roadmap. Chem. Eng. Res. Des. 177, 255–272 (2022).
Google Scholar
-
Tan, Y.-A. By-products of palm oil extraction and refining. OCL 13, 9–11 (2006).
Google Scholar
-
Oilseeds: World Markets and Trade (USDA Foreign Agricultural Service, 2023).
-
Eshel, G., Stainier, P., Shepon, A. & Swaminathan, A. Environmentally optimal, nutritionally sound, protein and energy conserving plant based alternatives to U.S. meat. Sci. Rep. 9, 103345 (2019).
-
Humbird, D. Scale-up economics for cultured meat. Biotechnol. Bioeng. 118, 3239–3250 (2021).
Google Scholar
-
Bajželj, B., Laguzzi, F. & Röös, E. The role of fats in the transition to sustainable diets. Lancet Planet. Health 5, 644–653 (2021).
Google Scholar
-
Román, S., Sánchez-Siles, L. M. & Siegrist, M. The importance of food naturalness for consumers: results of a systematic review. Trends Food Sci. Technol. 67, 44–57 (2017).
Google Scholar
-
Almroth, B. M. C. et al. Quantifying shedding of synthetic fibers from textiles; a source of microplastics released into the environment. Environ. Sci. Pollut. Res. 25, 1191–1199 (2018).
Google Scholar
-
Roser, M. Employment in Agriculture https://ourworldindata.org/employment-in-agriculture (2013).
-
World Bank: World Development Indicators (World Bank, 2022); https://databank.worldbank.org/data/reports.aspx?dsid=2&series=NV.AGR.EMPL.KD
-
Chen, C., Restuccia, D. & Santaeulàlia-Llopis, R. L. Land misallocation and productivity. Am. Econ. J. Macroecon. 15, 441–465 (2023).
Google Scholar
-
Michaels, G., Rauch, F. & Redding, S. Urbanization and structural transformation. Q. J. Econ. 127, 535–586 (2012).
Google Scholar
-
Pollin, R. & Callaci, B. The economics of just transition: a framework for supporting fossil fuel-dependent workers and communities in the United States. Labor Stud. J. 44, 93–138 (2019).
Google Scholar
-
Carley, S. & Konisky, D. M. The justice and equity implications of the clean energy transition. Nat. Energy 5, 569–577 (2020).
Google Scholar
-
Tzachor, A., Richards, C. E. & Holt, L. Future foods for risk-resilient diets. Nat. Food 2, 326–329 (2021).
Google Scholar
-
Socolow, R. et al. Direct Air Capture of CO2 With Chemicals: A Technology Assessment for the APS Panel on Public Affairs (American Physical Society, 2011).
-
Keith, D. W., Holmes, G., Angelo, D. S. & Heidel, K. A process for capturing CO2 from the atmosphere. Joule 2, 1573–1594 (2018).
Google Scholar
-
McQueen, N. et al. A review of direct air capture (DAC): scaling up commercial technologies and innovating for the future. Prog. Energy 3, 032001 (2021).
Google Scholar
-
Smil, V. Enriching the Earth: Fritz Haber, Carl Bosch, and the Transformation of World Food Production (MIT Press, 2004).
-
Foley, J. A. et al. Solutions for a cultivated planet. Nature 478, 337–342 (2012).
Google Scholar
-
Liu, J., Ma, K., Ciais, P. & Polasky, S. Reducing human nitrogen use for food production. Sci. Rep. 6, 30104 (2016).
Google Scholar
-
Dinerstein, E. et al. A ‘Global Safety Net’ to reverse biodiversity loss and stabilize Earth’s climate. Sci. Adv. 6, eabb2824 (2020).
Google Scholar
-
Turconi, R., Boldrin, A. & Astrup, T. Life cycle assessment (LCA) of electricity generation technologies: overview, comparability and limitations. Renew. Sustain. Energy Rev. 28, 555–565 (2013).
Google Scholar
-
House, K. Z. et al. Economic and energetic analysis of capturing CO2 from ambient air. Proc. Natl Acad. Sci. USA 108, 20428–20433 (2011).
Google Scholar
-
Yan, Z., Hitt, J. L., Turner, J. A. & Mallouk, T. E. Renewable electricity storage using electrolysis. Proc. Natl Acad. Sci. USA 117, 12558–12563 (2020).
Google Scholar
-
Friedlingstein, P. et al. Carbon budget 2022. Earth Syst. Sci. Data 14, 4811–4900 (2022).
Google Scholar
-
FAOStat (FAO, 2023).
Acknowledgements
S.J.D. was supported by the US National Science Foundation and the US Department of Agriculture (INFEWS grant EAR 1639318) and by the ClimateWorks Foundation (grant 22–2100). C.H. acknowledges support from the National Natural Science Foundation of China (42277087). These funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
I.M. and K.A. introduced S.J.D. to the idea of synthetic foods; S.J.D. conceived the analytical approach of the study and performed the analyses with support from K.A., M.S., K.C. and C.H.; S.J.D. and I.M. led the writing with input from all co-authors; J.M.-C. provided domain expertise in potential socio-economic impacts. All co-authors contributed to the interpretation of results and refinement of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
K.A. is an employee of Savor, a company that produces synthetic fats, and K.A. and I.M. have an ownership stake in the form of shares and/or options in the company. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Sustainability thanks Juan B. Garcia Martinez, Michael Clark and Karthish Manthiram for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Fig. 1, Discussion and Tables 1–8.
Reporting Summary
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
Reprints and Permissions
About this article
Cite this article
Davis, S.J., Alexander, K., Moreno-Cruz, J. et al. Food without agriculture.
Nat Sustain (2023). https://doi.org/10.1038/s41893-023-01241-2
-
Received: 08 February 2023
-
Accepted: 04 October 2023
-
Published: 06 November 2023
-
DOI: https://doi.org/10.1038/s41893-023-01241-2