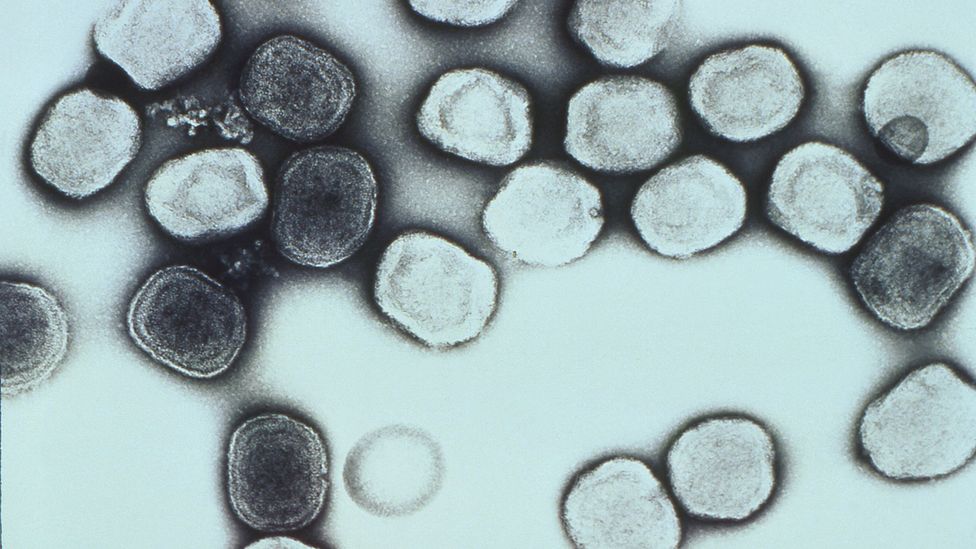
In the 16th Century, in the country now known as Mexico, there was a sudden and dramatic drop in the population. Disease spread after Europeans conquered the territory and millions of indigenous people died as a result. Until recently, it was believed the Europeans had brought the illness with them from Europe – but the exact pathogen responsible was unknown.
Now a team of scientists have extracted ancient viral DNA from the teeth of victims of the outbreaks, buried beneath a colonial-era hospital and chapel in New Mexico. The DNA reveals that the victims were infected with hepatitis B virus and human B19 parvovirus. Rather than a European origin as previously thought, the researchers found that the viruses likely originated in Africa.
“The viruses seem to have an African origin, and three of the humans we analysed were genetically African as well,” says María C Ávila Arcos, assistant professor at the International Laboratory for Human Genome Research at the Universidad Nacional Autónoma de México.
At the time, European traders and colonists were forcibly enslaving Africans and transporting them thousands of miles to the Americas. The cruel conditions on ships allowed infections to spread rapidly.
“These humans were kidnapped and then placed in crowded, unsanitary ships in completely subhuman conditions,” says Ávila Arcos.
“Once the virus arrived in the Americas, the indigenous people had never been exposed to it before, so they were extremely vulnerable. It wiped out a large fraction of the indigenous population.”
Rewriting history
The discovery is one of the latest in the field of paleomicrobiology, whereby researchers extract fragments of microbial DNA from centuries-old skeletons. The DNA is then reconstructed, allowing scientists to diagnose a disease or condition hundreds or even thousands of years after the person’s death. The technique is changing how we view and understand our past.
The origins of the virus which caused smallpox has been difficult to pin down (Credit: Getty Images)
Take smallpox – a devastating illness that killed an estimated 300 million people in the 20th Century alone. The origins of the variola virus that causes smallpox have always been obscure. From anecdotal accounts, historians believed it may have been around since 10,000BC, but until recently there was no scientific proof, and the oldest confirmed case was dated to the 17th Century using DNA extracted from a Lithuanian mummy.
However in 2020 that date was pushed back, when Barbara Muhlemann, a PhD student at the University of Cambridge, and her colleagues extracted variola virus from the teeth of Viking skeletons dated to AD600. The skeletons were uncovered in 11 burial sites in Denmark, Norway, Russia, and the UK. Smallpox was also found in multiple human remains from Öland, an island off the east coast of Sweden with a long history of trade. The finding predates the earliest confirmed smallpox cases by 1,000 years. The study provides clear evidence that the Vikings, who travelled extensively around Europe and beyond, carried smallpox with them.
Meanwhile ancient-DNA analysis has also shone a light on the origins of the plague. A 2015 study analysing microbial DNA from the teeth of 101 skeletons found that the bacteria that causes plague, Y. pestis, had been spreading amongst humans for at least 3,000 years prior to the first documented plague pandemic. The researchers found Y. pestis bacteria in the DNA of seven of the skeletons, the oldest of whom died 5,783 years ago.
Although modern plague is usually spread via rats carrying infected fleas, the study showed that the plague bacterium didn’t acquire the mutations necessary to infect fleas until the turn of the 1st millennium BC. Before then it likely spread through human-to-human contact, resulting in the less serious pneumonic plague. Once it acquired the ability to infect fleas it spread rapidly, leading to outbreaks of the bubonic plague (Black Death), a pandemic which wiped out half the population of Europe in the 14th Century.
Ancient DNA is changing the way we view another serious disease: syphilis. It was previously believed that the first outbreak of syphilis in Europe occurred in 1495 in Italy, just after Christopher Columbus returned from his first voyage to the Americas. The sexually transmitted disease ran rampant amongst the infantry of King Charles VIII of France, who at the time was planning to conquer the Kingdom of Naples. The debilitating disease then quickly spread around Europe.

The arrival of European colonisers in the New World also brought diseases which the local populations had never before encountered (Credit: Getty Images)
As the outbreak happened just after the return of Columbus and his crew from their first trans-Atlantic voyage, most historians believed syphilis spread to Europe from what was referred to at the time as the ‘New World’. But support is growing for a different theory.
In 2020, Verena Schuenemann, a professor of archaeological science affiliated with the University of Basel and University of Zurich, led a team which extracted DNA from nine individuals whose bones bore the characteristic lesions of syphilis. The remains were recovered from cemeteries in Finland, Estonia, and the Netherlands. The researchers detected at least three separate strains of Treponema pallidum, the pathogen that causes syphilis, as well as other diseases such as yaws – now found only in the tropics. Carbon-dating of the skeletons and coffins confirmed that the individuals died between the early-to-late 15th Century, suggesting that syphilis was already circulating in Europe before Columbus returned from his maiden voyage.
Calculations based on the mutation rate of the syphilis bacterium also suggest that the origin of the disease predates the time of arrival of Columbus.
“We know that the spread of pathogens is connected to trade routes,” says Schuenemann.
“We see that with syphilis, the plague, and leprosy – whenever humans started to exchange travel and trade, they also brought opportunities for pathogens to travel.”
Conversely, that means that microbial DNA can only tell us so much about the history of ancient pandemics. The DNA itself isn’t a problem. Although DNA does degrade over time, researchers have sequenced the genome of a woolly mammoth who lived one million years ago. However it’s likely that before around 12,000 years ago when humans adopted agriculture and farming, people wouldn’t have come into contact often enough to cause a pandemic.
“You need a certain amount of humans to actually transfer diseases, so we tend to see these diseases appearing alongside the first cities. When people started settling down, that’s when the pandemics struck,” says Schuenemann.
Clues from teeth
A great place to look for ancient microbial DNA is dental plaque. This sticky residue, which builds up on your teeth if you don’t brush properly, traps bacteria, eventually causing tooth decay and gum disease. Eventually plaque undergoes a mineralisation process whereupon it hardens, ‘trapping’ the DNA of ancient oral bacteria and viruses inside. Decoding this microbial genome is giving scientists access to a treasure trove of information about the health of our ancestors.
For example the multinational Medical project is using human dental plaque to piece together the history of how leprosy was treated during the Middle Ages in Europe. Led by Emanuela Cristiani from Sapienza University of Rome, the team have analysed the tartar of teeth excavated from the medieval cemeteries of St Leonard’s in Peterborough, England, and Saint-Thomas d’Aizier, in France.
You might also like:
- The ancient diseases that plagued the dinosaurs
- Leprosy: the ancient disease science can’t solve
- The viruses that fight human diseases
The team found traces of ginger in some individuals, suggesting that attempts were made to treat the condition. For instance, Constantine the African, a famous 11th-Century AD physician, wrote about preparing oral treatments containing ginger and other herbs to alleviate stomach pain triggered by leprosy. Mercury was also found in some patients, which could have been used to cover skin imperfections and as a pain-relieving ointment.
This suggests that rather than simply stigmatising sufferers, victims were cared for.
Diagnosing heart disease and Alzheimer’s
Sequencing dental DNA can do more than tell us about what infectious diseases a person had when they died. In the future, the technology could also reveal what a person’s oral microbiome – the huge and diverse assemblage of bacteria, archaea and fungi that live in and around your mouth – was like too.
This information, in turn, could tell us about the prevalence of noncommunicable diseases (NCDs) in ancient times. NCDs are chronic conditions that are not the result of a singular infectious agent. They include conditions like heart disease, diabetes, rheumatoid arthritis and Alzheimer’s Disease.
“There’s studies going back decades showing how oral health and the oral microbiome are related to these conditions,” says Abigail Gancz, a biological anthropologist at Pennsylvania State University.
In a recent article, Gancz argues that as the links between the oral microbiome and NCDs are so well-established, we may be able to use ancient microbial DNA to infer whether ancient human populations experienced these conditions too.
Although they are often thought of as modern diseases caused by unhealthy lifestyles, we actually have no idea to what extent they were prevalent in ancestral human populations, because the vast majority of NCDs leave no distinctive traces on the skeleton. However, there are signs that they were present, although not to the same extent as today.
“In ancient Egyptian literature in some of the earliest written records you have descriptions that look a lot like diabetes, for example accounts of the sweet-smelling nature of urine,” says Gancz.

The skeletons of victims of diseases such as bubonic plague – like these found in an east London cemetery – can still contain viral DNA centuries later (Credit: Getty Images)
“There is an assumption that people in the past lived short, brutal lives, but a lot of them did make it to older age and would have experienced these non-communicable diseases.”
Gancz is leading the Ancient Systemic Disease project, which aims to uncover the link between ancient human microbiomes and the presence of NCDs. So far, she has analysed the skeletons of 192 British individuals dating from the medieval to industrial period. The project involves painstakingly decoding the microbial DNA found in dental plaque to find out what bacteria and viruses the person had when they died. This is then compared to, and correlated with, known markers of disease present in the person’s skeleton.
“There are various lesions in skeletons that indicate disease,” says Gancz.
For example, cavities or abscesses in the person’s teeth may suggest that the person was suffering from periodontal disease. You can also detect signs of systemic inflammation by looking at a person’s bones. For example, arthritis can lead to a visual deformation in bone morphology.
Bones are living thing that change throughout the lifespan, so the bones will have different forms and textures depending on whether someone was actively experiencing stress,” says Gancz.
When complete, the research should allow scientists to look at a skeleton’s oral microbiome and use that information to calculate the likelihood that they also suffered from a chronic disease. This will allow researchers to track how conditions like arthritis and obesity have changed over time, including what effect if any the agricultural revolution, industrialisation, and urbanisation had on these conditions.
There’s good reason to believe that major transitions such as these had a huge impact on human health. For example, a 2013 study by Gancz’s supervisor, Laura Weyrich, associate professor of anthropology at Pennsylvania State University, found that the oral microbiome of our ancestors changed – and became more unhealthy – after we switched from hunter-gatherers to farmers.

Scientists have been able to decode the DNA of ancient animals preserved in bogs or ice – some up to a million years old (Credit: Getty Images)
The finding underlines the role that pathogens have had in shaping human history. But research like this isn’t just of historical interest. It could be used to improve the health of modern humans too. One of the aims of Weyrich’s research is to develop microbiome transplantations – swapping an unhealthy oral microbiome for a healthy one.
“Imagine a day where you don’t have to brush your teeth as you have microbes in your mouth that don’t cause oral disease – that might be something we can get to by looking at what microbes people had prior to the widespread presence of these modern oral diseases,” says Weyrich.
“I always tell people I wish I had teeth like a Neanderthal because the Neanderthals had brilliant oral health for the most part. If we could take their microbial composition and use that as a guide to develop modern oral microbial transplantation, we could try to fix the current situation we’re in where 90% of Americans will develop cavities throughout their lifetime.”
—
Join one million Future fans by liking us on Facebook, or follow us on Twitter or Instagram.
If you liked this story, sign up for the weekly bbc.com features newsletter, called “The Essential List” – a handpicked selection of stories from BBC Future, Culture, Worklife, Travel and Reel delivered to your inbox every Friday.