COVID-19 offers researchers their best chance yet to understand, and find treatments for, a chronic illness associated with an infectious disease.
Infection-associated chronic conditions are not widely understood. Partly because of this, people who develop such conditions often face scepticism and stigma; health-care systems are ill-equipped to deal with them; and cases are likely to be under-reported. When it comes to COVID-19, millions of previously healthy people were infected with SARS-CoV-2 at the same time, and many of those infections were confirmed through testing. Patient-advocacy groups and the media highlighted that symptoms persisted1 in some of these people, and governments globally started to invest in programmes to tackle what, by late 2020, was understood to be a widespread infection-associated chronic condition — long COVID.
Since the earliest days of the pandemic, we have been on the front lines of care, research and advocacy concerning long COVID — defined here as symptoms, such as cognitive dysfunction, fatigue, breathlessness and pain, that persist for months or years after SARS-CoV-2 infection. On many fronts, progress in the past three years has been impressive. But we are increasingly worried that the momentum will not hold.
Research on long COVID continues to be uncoordinated, with many researchers and clinicians communicating only with other experts from their own field, be they pulmonologists, neurologists or cardiologists, for example. Few clinical trials are testing interventions that address the root causes of the condition. And the lack of infrastructure for the rapid implementation of long COVID trials, as well as the absence of long-term resourcing for the field, means that many scientists and companies with potential therapeutics are hesitating to engage. Meanwhile, the world is desperate to move on from COVID-19 — even as there is another global uptick in the spread of SARS-CoV-2.
We call for a moonshot for long COVID, a commitment — from the US government — to invest at least US$1 billion annually over the next ten years to address the problem. Such an investment would inspire governments around the globe to respond in kind to this health challenge, which exists on every continent.
There are precedents for this. In 2016, the US Congress directed more than $1.8 billion towards cancer research through the 21st Century Cures Act. Among the various efforts funded was the Cancer Moonshot initiative, supporting more than 240 research projects, many of which focused on improving understanding of cancer biology. Since then, billions more have been proposed for cancer research across multiple initiatives. Meanwhile, across the US National Institutes of Health (NIH), more than $3 billion each year is spent on improving testing and treatment for HIV/AIDS, and driving progress towards a cure.
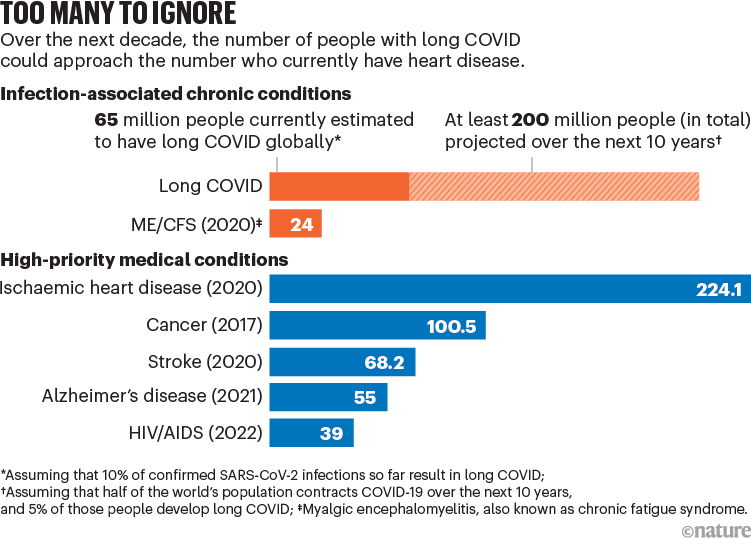
Such an investment will more than pay itself back. According to the US Centers for Disease Control and Prevention, around 6% of US adults are experiencing symptoms following a SARS-CoV-2 infection that do not resolve for months or years, and that might not resolve at all. Other studies have estimated that 18 million adults in the United States2 and 65 million people globally3 have long COVID (see ‘Too many to ignore’). Children are also affected. For the United States alone, the economic cost of long COVID is estimated to be $3 trillion over the next five years4.

SourceS: long COVID (current); H. E. Davis et al. Nature Rev. Microbiol. 21, 133–146 (2023); ME/CFS; ischaemic heart disease & stroke; cancer; Alzheimer’s disease; HIV/AIDS
Besides reducing economic costs, identifying the biological differences between people with long COVID and those who recover from COVID-19 with no lasting effects, and probing these pathways to establish treatments, could improve the lives of tens, if not hundreds, of millions of people globally. This includes people who have other infection-associated chronic conditions. It could lessen morbidity and disability for generations to come, and prompt both researchers and clinicians to think about infectious diseases in a very different way.
Progress so far
Infection-associated chronic conditions, in which infections cause debilitating symptoms that persist for months, years or indefinitely5, are under-studied. There are no well-accepted measures or biomarkers for diagnosing them. Although shared biological mechanisms almost certainly underlie these conditions, none has yet been identified. And no treatments targeting root causes, rather than symptoms, have been approved by regulators.
Yet even before long COVID, such conditions were highly prevalent. In 2020, up to 2.5 million people in the United States and 24 million globally had myalgic encephalomyelitis, also known as chronic fatigue syndrome6, which has been linked to herpesvirus and enterovirus infections. In 2020, up to 1.9 million people in the United States alone might have had post-treatment Lyme disease, in which neurological symptoms, fatigue and other effects persist even after treatment of the infection7. Meanwhile, muscle aches, fever, fatigue and other problems that persist for up to two years following infection have been reported by up to 75% of Ebola survivors8 (mainly in Sierra Leone and Liberia), and diarrhoea and fatigue that persist for up to a decade by as many as 40% of people infected with the parasitic microorganism Giardia duodenalis9.

A person in Brazil with long COVID undergoes a physical assessment.Credit: Silvia Izquierdo/AP Photo/Alamy
So far, long COVID has been an outlier in terms of the amount of attention it has received compared with other infection-associated chronic conditions, which have historically garnered little attention relative to disease burden. Thanks in part to teams studying other conditions, such as HIV and Ebola, being able to redirect resources towards long COVID, various research and clinical programmes to address it were established in 2020 — including at the University of California, San Francisco, where one of us (M.J.P.) is directing a long COVID research programme10. Today, epidemiological studies are under way to better establish how many people are affected, who is affected and to what degree. Larger initiatives, such as the NIH Researching COVID to Enhance Recovery (RECOVER) programme, launched in 2021, are building on the smaller efforts that jump-started the field, including one of our own organization’s (L.M.’s) patient-led research studies. And biological mechanisms and potential therapeutic targets are beginning to emerge.
It is now known, for instance, that one of at least six mechanisms (or any combination of these) could be contributing to the persistence of symptoms long after a person has been infected with SARS-CoV-23.
Multiple studies have identified inflammation markers, both in blood11 and in tissues12. Others have found that the Epstein–Barr virus, normally dormant in adults, can be reactivated in people with long COVID3. Researchers in the United States, South Africa and the United Kingdom have shown that SARS-CoV-2 can alter clotting proteins, generating microscopic clots that might affect tissue function3. Some people experience autoimmune conditions after getting COVID-19, suggesting that a contributing factor to long COVID could be the immune system attacking the body3. SARS-CoV-2 can alter mitochondrial health3, offering a potential explanation for why many people with long COVID experience fatigue and post-exertional malaise. Even the idea that SARS-CoV-2 is a transient invader is being challenged13, with multiple studies showing that fragments of the virus can stay in the body for more than six months.
Many questions remain. Yet acquiring even this understanding in such a short time suggests that precisely defining long COVID’s biology, identifying biomarkers and designing and testing targeted treatments should be possible — so long as the funding is there.
Six priorities
We estimate that by this point, more than $1.5 billion globally has been dedicated to long COVID research. But most of this funding has been limited to a maximum of four years. Building on the progress made so far is going to require billions more, and for resources to be committed over at least a decade. It will also need funders, regulators, researchers, pharmaceutical companies, the patient community and other stakeholders to prioritize six goals.
Agree on what long COVID is. Studies of long COVID define the condition in different ways. Some investigators include medical comorbidities associated with COVID-19, from diabetes and cardiovascular disease to Alzheimer’s disease. In some studies, symptoms must have lasted for at least three months; in others, for just 30 days. Various attempts at standardization have been made or are still under way, including efforts by the World Health Organization and the US National Academies of Sciences, Engineering, and Medicine. But most people working on long COVID continue to use the definition that is most convenient for them. This means researchers are comparing apples to — at best — oranges. The lack of clear definitions also feeds scepticism over whether the condition can even be studied in the first place.
For now, research funders and publishers could encourage more consistency by requiring that, at the very least, researchers state which formal definition they are using in their funding applications and manuscripts.

A woman undergoes oxygen therapy for chronic symptoms after contracting Lyme disease.Credit: Philipp Guelland/Getty Images for The Herald Sun
The clarity of a single, formal definition might be unobtainable. Definitions developed in the coming years could require more specific patient data, and might not easily be applied to parameters captured in 2020 or 2021. Also, it’s unclear whether a single definition can be applied across cohorts, such as those who were hospitalized with COVID-19 and those who were not. The population of people with long COVID but without a positive COVID test, either because their test was inaccurate or because they weren’t able to obtain a test, is clinically important and must also be accounted for. So it could be that a set of case definitions with varying degrees of specificity will be most useful. In clinical settings, a broad definition of long COVID could ensure that people are eligible for care. In research, a narrower definition might be warranted, although investigators should strive to ensure that any definition they use is as inclusive as possible.
Bolster team science. The first major international multidisciplinary meeting focused on long COVID — the Keystone symposium on long COVID — was held in Santa Fe, New Mexico, this August. This is exactly the kind of event needed to bring together clinical scientists, infectious-disease experts, neuroscientists, cardiologists, pulmonologists and many other researchers (including patients themselves) studying the mechanisms that might be at work in people with long COVID.
Various efforts have already been made to bring together different groups working on long COVID. Foundations and patient groups, for instance, are hosting seminars and connecting people online. But the creation of publicly funded multidisciplinary centres specific to infection-associated chronic conditions, including long COVID, would help to drive much more collaboration.
In the United States, the creation of an NIH Office for Infection-Associated Chronic Conditions Research would help to coordinate research across organizations. This would be similar to the Office of AIDS Research, which has budgetary authority and ensures the coordination and prioritization of HIV/AIDS research across the NIH. Equivalent institutions could be established in other countries.
Curate resources from the ‘pristine period’. Studying long COVID is more complicated now than in the first 18 months of the pandemic. Back then, researchers didn’t have to factor in the emergence of new variants or vaccines and treatments. Testing for SARS-CoV-2, although not accessible or accurate for all, became widely available in many countries by mid-2020. And because people wore masks and isolated from others, few other pathogens were circulating. By contrast, researchers are now trying to study long COVID against a backdrop of several circulating SARS-CoV-2 variants; reinfections that often go undiagnosed; and variability in the number, timing and type of SARS-CoV-2 vaccine doses people have had.
Fortunately, in 2020, some groups were well-positioned to establish clinical and biospecimen repositories. What’s needed now is an initiative to ensure that all the samples collected during this ‘pristine period’ are properly catalogued, and to facilitate data sharing among researchers. This effort could be similar to the various centralized repositories established for acute COVID-19 during the early months of the pandemic — such as those set up by the European Biobanking and BioMolecular Resources Research Infrastructure.
Accelerate biomarker discovery. Although we don’t think researchers and drug developers should wait for biomarkers to be identified and validated before forging ahead, the discovery of reliable biomarkers for long COVID would be transformative. Researchers are unlikely to find a universal biomarker because multiple mechanisms seem to be at play, and different combinations of these could be involved in different people. Yet consistent and measurable abnormalities have already been documented. Several teams, for example, have identified SARS-CoV-2 proteins in the blood of people with long COVID, which might come from the virus persisting in certain tissues13. Other parameters, such as a person’s capacity to exercise14, are also being evaluated. Eventually, combinations of biomarkers might prove most useful15.
Ultimately, biomarkers should be validated using samples from multiple large-scale collections, such as those amassed by RECOVER, or specimens collected during the ‘pristine period’. Such validation efforts could be coordinated by our proposed Office for Infection-Associated Chronic Conditions Research.
Invest in experimental medicine. A search for ‘long COVID’ in the registry ClinicalTrials.gov (which holds information on more than 400,000 trials worldwide) returns some 412 trials. Of 240 classed as interventional, only 91 are recruiting. And only 12 are testing pharmacological interventions rather than interventions that focus on behavioural modification or rehabilitation16.
Big pharmaceutical companies are currently on the sidelines, awaiting clarity on long COVID case definitions and biomarkers before pursuing clinical trials. Meanwhile, many small, less-risk-averse biotech companies lack the resources for drug development. (It takes ten years, on average, to bring a drug to market17.) Although foundations and private donors have helped to fill the gap, in our view, a US Office for Infection-Associated Chronic Conditions Research and equivalent bodies elsewhere should establish high-risk, high-reward funding mechanisms that incentivize many more investigators, including from industry, to conduct long COVID clinical trials — especially proof-of-principle trials. Such trials might involve just 10–50 people and are not designed to demonstrate the efficacy of a drug. But they can use existing drugs to probe the effects of intervening in various biological pathways18 — and, by driving the science, help to accelerate the discovery of treatments.
Using foundation funds, one of us (M.J.P.) is conducting a proof-of-concept trial to investigate whether monoclonal antibodies can clear SARS-CoV-2 from tissues that retain the virus. Other potential therapeutics that could be trialled include drugs that modify the immune response (immunomodulators) or that stop blood clotting (anticoagulants). In the United States, people with long COVID have even presented the Food and Drug Administration (FDA) with a list of potential therapeutics to trial, chosen on the basis of lived experience.

A researcher accesses stored blood samples as part of a long COVID study.Credit: Lisa Maree Williams/Getty
A key challenge is that no rapid funding mechanisms are available to take these and other promising leads forwards. Unless they use foundation money or discretionary funds, researchers face review times for proposals of 6–12 months. Even once a project has been funded, it can take more than a year before a trial can begin. Added to this is the lack of clarity around what regulatory requirements need to be fulfilled for clinical trials to be approved. What data will regulators require to assess people’s eligibility for such a trial, for instance? Will patient-reported measures suffice for a regulator to approve a potential treatment?
Agencies such as the FDA or the European Medicines Agency could take steps to expedite the review of treatments intended to treat long COVID — just as they did for those for acute COVID-19. In the United States, ‘fast track’ or ‘breakthrough’ therapy designations — used for interventions for people with serious unmet medical needs or drugs that could be substantially better than existing ones — could be applied. Likewise, until researchers identify reliable biomarkers for long COVID, regulators could allow them to use patient-reported measures of symptoms and quality of life.
Link research efforts to coordinated clinical programmes. In 1991, the Ryan White CARE Act made more than $220 million in federal funds available for HIV care for low-income, uninsured and under-served people throughout the United States. Since then, the amount spent on this has grown to more than $2 billion. For more than 30 years, this programme has reduced stigma, provided treatment annually to more than 500,000 largely marginalized people and trained thousands of providers to deliver HIV care.
Long COVID is likely to have most impact on those populations that have been disproportionately affected throughout the pandemic — communities of colour, with less access to health care, and a higher prevalence of comorbidities19. Similar coordinated clinical programmes for long COVID and other infection-associated chronic conditions could help those most in need and make it easier for diverse populations seeking care to get involved in research efforts. Such programmes, which are beginning to be established, would help to ensure that any advances in treatments are transferred to the clinic equitably.
Holistic response
Investment now in long COVID will help to ensure that when the next pandemic comes, efforts to study potential infection-associated chronic conditions are a core part of the initial response. That means investing not just in research, but also in education programmes that enable health professionals and trainees to learn about such conditions. And it means providing grant funding, training programmes and salaries specifically for early-stage investigators wanting to pursue careers in such conditions, including long COVID.
By challenging the long-held assumption that treating infectious disease is all about tackling acute infection, a moonshot initiative for long COVID could ultimately change the way all of us think about the effects of pathogens on our health.