Abstract
Recent studies have indicated potential health risks associated with microplastics (MPs) exposure, including alterations in blood coagulation homeostasis. This cross-sectional study aimed to quantitatively examine MPs in human blood and assess their association with coagulation markers. We recruited 36 healthy adults, collected whole blood samples, and analyzed MPs using Fourier-transform infrared (µ-FTIR) spectroscopy. Lifestyle factors related to MP exposure were assessed, such as the use of plastic food containers. Coagulation and inflammatory markers in blood samples were analyzed, including C-reactive protein, prothrombin time, activated partial prothrombin time (aPTT), antithrombin III, platelet count, erythrocyte sedimentation rate, and fibrinogen. MPs were detected in 88.9% of the participants, with a mean concentration of 4.2 MPs/mL. The predominant types of plastics identified were polystyrene and polypropylene. MPs were significantly higher in participants with a greater use of plastic food containers. A high MP load in the blood (≥ 3 MPs/mL) was significantly correlated with increased aPTT, C-reactive protein, and fibrinogen. We identified MPs in human blood, their association with specific lifestyle factors, and significant alterations in coagulation markers. This underscores the need for strategies to reduce human exposure to MPs, particularly in relation to blood coagulation and potential cardiovascular risks.
Similar content being viewed by others

Micro-mechanical blood clot testing using smartphones
Lifestyle and demographic associations with 47 inflammatory and vascular stress biomarkers in 9876 blood donors
Fibrinogen and hemoglobin predict near future cardiovascular events in asymptomatic individuals
Introduction
Plastics are synthetic or semi-synthetic organic polymers with high molecular weight, derived from the Greek word “plastikos,” meaning moldable. Plastics are primarily generated by polymerizing monomers derived from coal, gas, or oil. Due to their affordability, ease of processing, and chemical stability, plastics have dramatically increased over time—from a global production of approximately 1.5 million tons in 1950 to about 390 million tons in 20211. According to a 2016 report by the Ellen MacArthur Foundation presented at the Davos World Economic Forum, plastic usage has increased by approximately 20 times over the past 50 years. This concerning trend suggests that by 2025, the ratio of trash to fish in the ocean could be 1:3, leading to more plastic than fish in the oceans by 20502.
The term “microplastic” (MP) was coined in 2004 by Dr. Thompson from the UK, who observed the accumulation of microscopic plastic fragments and fibers in marine environments3. Microplastics are generally defined as plastic particles smaller than 5 mm—a definition established during a 2008 international workshop hosted by the National Oceanic and Atmospheric Administration (NOAA)4. These fragments, including those identified in marine sediments using Fourier-transform infrared (µ-FTIR) spectroscopy, exemplify the growing concern regarding microplastic pollution. Plankton samples collected routinely in the 1960s from the northeast Atlantic near the UK revealed an increasing presence of microplastic particles, correlating with global synthetic fiber production. MPs are categorized into primary microplastics, which are artificially manufactured to be less than 5 mm in size, and secondary microplastics, which are formed from the fragmentation of larger plastics due to environmental factors such as UV radiation, marine activity, and biological attachment. Secondary MPs and nanoplastics are generally produced by the breakdown of macroplastics via shear forces5.
The human body is exposed to MPs through ingestion, inhalation, and skin exposure6. MPs are present in food, drinking water, and food packaging7. Products closely associated with daily life, such as plastic teabags8, feeding bottles9, and snacks10 have been reported to contain MPs. MPs have been investigated in various human biological samples, including colectomy specimens11, saliva12, sputum13, lungs14, liver15, breast milk16, and feces17. The detection of MPs in systemic tissues suggests that MPs may also be detected in the bloodstream, which supplies the entire body.
Few studies have reported on the presence of MPs in human blood18,19,20,21. In vivo studies have reported that MPs can cause physical toxicity, oxidative stress, inflammation, immune reactions, and an additional burden of plastic additives22. Systemically, these particles can have detrimental effects on cardiovascular systems23. Some animal studies have reported that MPs can alter thrombosis formation and coagulation factors24,25. However, the epidemiologic study on this issue has been scarce. More recently, a striking report has indicated that the presence of MPs in human atheroma is associated with the incidence of cardiovascular events26. This study suggested that human exposure to MPs could be associated with coagulation function; however, no method for assessing MP exposure using human blood or studies employing this assessment has been investigated. Therefore, this study aimed to quantitatively examine microplastics in human blood and investigate the association between these findings and health indicators, including coagulation markers.
Materials and methods
Study participants
A total of 36 healthy adults were recruited for the study through notices posted at Inha University Hospital, Korea, from October 30, 2023, to November 7, 2023. The inclusion criteria were: (1) age between 20 and 60 years, and (2) provision of informed consent. Exclusion criteria were: those who had (1) chronic liver diseases (e.g., Hepatitis B or C virus carrier or liver cirrhosis), (2) previous history of coronary angioplasty and stenting due to coronary disease, (3) chronic kidney disease (estimated glomerular filtration rate < 60 ml/kg/1.73 m2), (4) any type of cancer, and (5) any psychiatric disorders. The sample size was determined based on Johanson and Brooks’ recommendation of 30 participants for a preliminary study, with a predicted dropout rate of 20%27. This study was approved by the Institutional Review Board of the Inha University Hospital (2023-05-016). This study was investigated in compliance with the Declaration of Helsinki. All the participants provided written informed consent.
Questionnaires
Information on participants’ demographics and plastic-related lifestyles was obtained using questionnaires that included the following items: sex, age, education (high school graduate, college graduate, or post-college), job (white-collar vs. blue-collar), marital status, smoking status (never, former, or current smoker), alcohol consumption, and physical inactivity. Physical inactivity was defined as moderate-to-vigorous physical activity for less than 150 min/week28. The plastic-related lifestyle questionnaires were screening questionnaires for environmental health clinics at Seoul National University Hospital, developed based on the references29,30. The questionnaire assessed the frequency of having ready-made meals (≥ 1/week or < 1/week), percentage of plastic containers among total containers in the refrigerator (≥ 50% or < 50%), percentage of discolored plastic container among total plastic containers (≥ 25% or < 25%), frequency of consuming vinyl-containing food (≥ 1/week or < 1/week), the frequency of consuming seafood (≥ 1/week, or < 1/week), and the frequency of indoor ventilation (≥ 1/day, < 1/day).
Blood sampling
Whole blood was obtained via venipuncture of the antecubital veins. Approximately 25.7 ml of blood was collected into a BD Vacutainer (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) 366,480 Sodium Heparin Glass tube, followed by Serum SST, EDTA, and Sodium citrate tubes. Samples in glass tubes for µ-FTIR analysis were stored at − 20 °C in a refrigerator for up to one week and then transferred to the Korea Institute of Analytical Science and Technology (KIAST) for µ-FTIR spectroscopy. KIAST conducted background contamination tests on the entire sampling system, including the needle and glass tubes. Blood samples were stored in a freezer at − 20 °C until analysis, for at least one week and at most eight weeks.
Extraction method
Preparation and analysis by µ-FTIR were performed by KIAST (https://www.kiast.co.kr/eng/main/main.php), an institute certified by the Korea Laboratory Accreditation Scheme (KOLAS). To remove organic matter from the blood, 30% H2O2 was added in 1 mL increments, carefully monitoring its rapid reaction with the blood. The total amount of added H2O2 was 30 mL. The mixture was shaken at 60 °C and 110 r/pm using a shaking incubator (Hanbaek Scientific Technology, HB-201SF) for a total of 2 weeks. Each blood sample (1 g) was placed in a prewashed flask using a glass Pasteur pipette. To remove organic matter from the blood, 30% H2O2 was added, and the mixture was shaken at 60 °C and 110 r/pm using a shaking incubator (Hanbaek Scientific Technology, HB-201SF). After adding 1 ml of 68% HNO3, the solution was filtered through a 5 μm silicon filter (10 mm × 10 mm square filters, pore diameter 5 μm, SmartMembrane, Germany). The flask was then washed with ultrapure water and filtered.
Analysis by µ-FTIR
One of the challenges in studying the health effects of microplastics on humans is exposure assessment31. Although markers indicating the exposure levels of MPs have been suggested, related studies are scarce. The samples were measured using µ-FTIR spectroscopy with a microscope (LUMOS II, Bruker Optics, USA) equipped with a 32 × 32-pixel focal plane array (FPA) detector. Infrared (IR) images were acquired in transmission mode with a spectral resolution of 8 cm− 1 and a single scan over the spectral range of 4000 to 750 cm− 1. The filter surface was obtained in Fig. S1a. Before IR imaging, sample photographs were taken to visualize surface morphology. Data analysis was performed using siMPle software, a freeware capable of rapidly detecting microplastics32. This software performs analyses using an algorithm that compares the IR spectrum of the sample with each reference spectrum in the database and assigns materials based on probability scores. The spectral library used in the analysis comprised spectra from polypropylene (PP), polyethylene (PE), polyethylene terephthalate (PET), polystyrene (PS), polyvinylchloride (PVC), polyurethane (PU), polyamide (PA), poly(methyl methacrylate) (PMMA), and polycarbonate (PC). Plastic materials were classified into various plastic groups using the siMPle software. Samples with a major-to-minor axis ratio greater than 3 were classified as fibers, while the remaining samples were classified as fragments. The major diameters of the samples were used to classify them into five size classes: 5–10 μm, 10–20 μm, 20–50 μm, 50–100 μm, and > 100 μm.
Scanning electron microscopy
The morphology of particles collected on silicon filters was characterized using scanning electron microscopy (SEM) (JEOL JSM 7001 F, Japan). Before observation, the silicon filters were dried at 60 °C and sputter-coated with 7–10 nm of platinum to enhance image clarity. Images were acquired at an accelerating voltage of 15 kV, with magnifications ranging from 500 to 10,000 times, allowing for both overview and detailed examinations of the particles.
Analysis by µ-Raman
For the sample where MPs were not detected by µ-FTIR, a µ-Raman analysis was performed using an XploRA Plus confocal Raman microscope (Horiba, France) to verify blood samples that were not detected using µ-FTIR. A 532 nm laser with 10% decreased power and a 1024 × 256 pixel cooled charge-coupled device (CCD) detector were used. Grating with 1200 grooves/mm, a 100 μm confocal hole, and a 50 μm confocal slit width were set. A mosaic of microscopic dark field images of the filter surface was obtained, as illustrated in Fig. S1b. The mosaic image was processed using the Particlefinder™ module by Labspec 6 software, and all bright particles were selected against the dark background area. Raman spectrum was recorded by accumulating 1 × 2 s exposure time with a spectrum range between 1020 and 3400 cm1. A baseline correction of collected spectra was conducted using Labspec6 software. All spectra were screened for plastics using a classical least square algorithm (CLS). Each measured spectrum was calculated as the sum of all references and the theoretical composition. The results of the CLS Spectra were investigated manually to avoid missing or false positives by know-it-all software spectrum matching software with a library of Raman.
Quality control
To prevent microplastic contamination, all plastic tools were excluded, and glass and metal materials were used during the sampling and filtering processes. All glassware was washed with ultrapure water before use, and chemical reagents were used after being filtered through a 0.5 μm metal filter (KIAST, Korea). To prevent airborne microplastic contamination, sampling, pretreatment, and filtration steps were conducted on a laminar flow bench (HSCV-1300, SINAN Scientific Industry, Korea) inside a laminar flow cleaning booth. All samples were covered with aluminum foil when moved outside the laminar flow bench. Nitrile gloves and cotton coats were used to minimize sample contamination. Control samples were prepared for each sample set and pretreatment step to perform the same protocol and assess the potential impact of contamination.
To evaluate the potential loss and damage that may occur during pretreatment, the recovery rate and surface morphology before and after pretreatment were compared using the same processes as those of the sample. Reference materials (RMs) of different sizes and types were used to distinguish between the intentionally added microplastic particles and those in the blood medium. Before spiking, RMs were dropped on the sliding glass to an appropriate number to manually count and confirm the shapes using a micro-Raman microscope and then injected into the blood. Fiber-shaped PP greater than 100 μm, average size of 27 μm PS sphere, and average size of 65 μm PS sphere were used for recovery testing. PET fiber greater than 100 μm was additionally added for damage testing. After the treatment of RMs spiked samples, the number was manually counted, comparing the FPA chemical image of µ-FTIR and the Raman microscopic image. Surface morphology was analyzed using an SEM (JEOL JSM 7001 F, Japan) to determine whether the chemical reagents damaged the microplastics after pretreatment. Surface morphology was analyzed using an SEM to determine whether the chemical reagents damaged the microplastics after pretreatment.
To evaluate potential contamination that may occur during the pretreatment of microplastics, procedural blank tests were conducted. A total of five blank samples were prepared when the sample batch was pretreated. Procedural blank samples that did not contain blood samples were treated using the same process as those used for blood samples. The results of the blank test were not subjected to any subtraction or correction but were intended to provide information regarding the environmental contamination of microplastics detected in the blood samples in this study.
Coagulation and inflammation markers
Blood collected using a Vacutainer Sodium Citrate tube was used to analyze prothrombin time (PT) and activated partial prothrombin time (aPTT). Fibrinogen was measured using an STA analyzer (Diagnostica Stago, Asnieres-Sur-Seine, France) within 4 h after blood collection. Antithrombin III activity was determined by an ELISA using assay kits (Diagnostica Stago). Blood collected by EDTA-anticoagulated Vacutainer Tube was used for platelet counts, which were measured using an automated analyzer (ADVIA 120; Siemens, Forchheim, Germany). Serum high-sensitive c-reactive protein (hsCRP) levels were measured by an immunonephelometric assay (Dade Behring Inc., Deerfield, IL, USA). StaRRsed Auto-Compact (Mechatronics Manufacturing BV, Zwaag, Netherlands) was used to measure the Erythrocyte sedimentation rate (ESR) with the Westergren sedimentation technique.
Statistical analysis
The differences in the detection of MPs and the number of total MPs by the characteristics of the participants and plastic-related lifestyle were tested using the Wilcoxon rank-sum test (or Kruskal-Wallis rank-sum test) and Chi-squared test, respectively. To compare the differences in the coagulation and inflammation markers by MP levels in blood, we divided the samples into two groups based on total MP particle counts: low (< 3/ml) and high (≥ 3/ml), according to the median value. Differences in hsCRP, PT, aPTT, antithrombin III, platelet count, ESR, and fibrinogen between the two groups were tested using the multivariate linear regression models with the adjustment for gender, age, education, job, marital status, smoking, alcohol, and physical inactivity. Additionally, we examined the beta coefficients (β) and P-values of the linear regression models between the number of total MP particles in the blood and each marker. All statistical analyses were performed using R version 4.2.3.
Results
Table 1 presents the characteristics of the study participants and the number of MP particles. Among the 36 participants, 10 (27.8%) were male and 26 (72.2%) were female. The median age was 41 years, with the range of 30–55; 17 (47.2%) participants were younger than 40 years, while 19 (52.8%) were 40 years of age or older. Most of the participants (N = 21, 58.3%) were college-educated, followed by post-college (N = 14, 38.9%) and high school graduates (N = 1, 2.8%). Most participants had white-collar jobs (N = 32, 88.9%) and were married (N = 26, 72.2%). Participants who were nonsmokers (N = 33, 91.7%), consumed alcohol (N = 27, 75.0%), and were physically inactive (N = 24, 66.7%) were more common than former smokers (N = 3, 8.3%), participants who did not consume alcohol (N = 9, 25.0%), and those who were physically active (N = 12, 33.3%), respectively. The number of samples in which MPs were detected (qualitative) and the number of MP particles (quantitative) in samples were counted according to their respective characteristics. There were no significant differences in the total MP counts in whole blood according to sex, age, education, job, marital status, smoking, alcohol consumption, and physical inactivity. Participants with post-college degrees showed a significantly higher number of MPs (5.7 MPs/ml) in their blood samples than those who graduated from college or had lower levels of education (2.0 MPs/ml). Detailed information on the classification and distribution of the MPs is provided in Supplementary Tables S1–2.
Among the 36 whole blood samples collected from participants, MPs were detected in 32 samples using µ-FTIR. The microphotographs and corresponding FTIR spectra of some selected MPs found in the blood samples are illustrated in Fig. S2. The procedural blank test results (Fig. S3) showed that 0 to 5 microplastic particles were detected in the blanks, suggesting that an average of 1.67 ± 2.12 particles have a possibility of contamination in the samples. In this study, no subtraction or correction using blank values was applied during the statistical analysis. The average MP concentration was 4.2 MP counts/1 ml (Table 2). The most detected plastic was PS (58.3%), followed by PP (50.0%), PE (38.9%), PET (16.7%), and PA (8.3%). MPs in 20–50 μm were most commonly observed in 75.0% of all samples, and MPs in 10–20 μm, 50–100 μm, ≥ 100 μm were detected in 52.8%, 19.4%, and 16.7% of all samples, respectively. Fragment MPs (86.1%) were more commonly detected than fiber MPs (36.1%). Figure 1 depicts the SEM images of selected MP particles in human blood samples. No morphological deformation was observed before and after the organic matter removal treatment, indicating that the microplastics were not damaged by H2O2 and HNO3 during the blood pretreatment process.
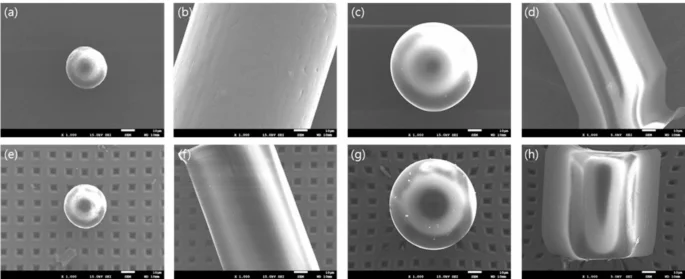
SEM images of (a) PE, (b) PP, (c) PS, and (d) PET in the pristine state and (e) PE, (f) PP, (g) PS, and (h) PET after organic matter removal treatment.
Table 3 demonstrates the detection and number of MPs according to lifestyle-related factors. The frequency of consumption of ready-made meals, vinyl-containing food, and seafood, as well as the level of indoor ventilation, did not exhibit significant differences in the detection of MPs or the mean number of MPs detected. The percentage of plastic containers among the other packaging in the refrigerator was significantly associated with the number of MPs, with the half or more group reflecting an average of 6.8 MP particles/ml, while the less than 50% group recorded an average of 2.4 MP particles/ml.
Coagulation-related markers differed significantly in the high-MP particles group (Fig. 2; Table 4). The aPTT was significantly prolonged in the high-MP group compared with the low-MP group (mean ± SD; 35.75 ± 2.67 vs. 33.78 ± 2.87 s, respectively; P = 0.037). The difference between platelet count between the high-MP group and the low-MP group (mean ± SD; 35.75 ± 2.67 vs. 33.78 ± 2.87 × 103/µl, respectively, P = 0.081) was marginally significant. Significant linear associations were observed between MP particle counts and hsCRP (β = 0.050, P < 0.001), as well as between MP particle counts and fibrinogen (β = 4.260, P = 0.036).
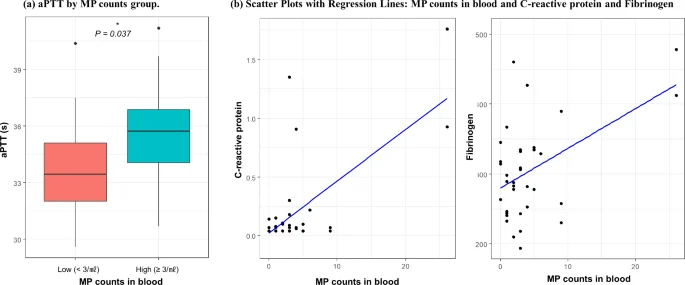
aPTT by MP counts group, and scatter plots with regression lines: MP counts in blood and C-reactive protein (hsCRP) and fibrinogen. * The P-value was calculated using multivariate linear regression models adjusted for gender, age, education, job, marital status, smoking, alcohol, and physical inactivity; MP microplastic, aPTT activated partial platelet time.
Additionally, four samples with no microplastics detected using µ-FTIR were evaluated using µ-Raman. As a result, two samples had microplastics, while two samples still did not contain microplastics.
Discussion
In this study, we detected MPs in the blood samples of 32 out of 36 participants through µ-FTIR. The four samples with no MPs detected were subsequently identified using µ-Raman methods, and MPs were found in two samples. The mean MP particle concentration in blood, as measured by µ-FTIR, was 4.2/mL, with most particles ranging in size from 20 to 50 μm. The number of MP particles in blood was higher among higher education levels, and the percentage of plastic containers among total vessels in the refrigerator. Higher MP particle concentrations were associated with altered coagulability. Compared to the low-MP group (< 3/ml), the high-MP (≥ 3/ml) group showed a significantly longer aPTT. Furthermore, positive associations between MP particle counts and hsCRP and MP particle counts and fibrinogen were significant.
Recently, Leslie et al. reported MP particles in human blood from 22 healthy volunteers18. The prepared samples were filtered through a glass fiber filter with a mesh size of 700 nm, and the samples were analyzed by pyrolysis-gas chromatography-mass spectrometry (Py-GC/MS). Seventeen of the 22 participants had a quantifiable mass of plastic particles in their blood samples. However, the size of MP particles in the bloodstream could not be reported in this study because the analysis method could not provide this information. Furthermore, the differences between duplicated measurements of the same samples imply that the results were unstable and unreliable. More recently, MPs were detected in 8 out of 20 health volunteers using µ-FTIR with the mean concentration of MP particles was 2.4/mL33.
In our study, most detected MPs’ lengths in human blood samples were in the range of 20–50 μm. Due to the detection limit of the length of µ-FTIR, smaller-sized MP particles could exist in the blood samples. A particular finding of this study is that relatively large MP particles were found in blood samples. Regarding the human uptake of microplastics, MP particles < 10 μm can cross cellular membranes and reach organs, but MP particles < 150 μm are assumed to pass the gastrointestinal barrier34. However, MP particles larger than 20 μm are not surprising, as this finding is consistent with previous animal and human studies. Deng et al. orally administered 5 μm and 20 μm of PS MP particles to mice and found that they accumulated in the liver35. Wei et al. detected MP particles in the blood of mice after a single administration of 3 μm PS beads via gavage36. Hussain et al. reported the detection of various sizes of MPs in the range of 0.1–150 μm in the gastrointestinal lymphatics of various species, including rodents, dogs, rabbits, and humans37. A study investigating MP particles in human saphenous vein samples showed a mean particle length of 119.59 ± 226.82 μm (range 16–1074 μm)38. Another study reported the detection of MP particles up to 29.5 μm in human liver tissue, within the range of 4–30 μm of detection level15.
One potential route for the uptake of MP particles of several micrometers in size is through persorption. This phenomenon was proposed by Volkheimer based on several studies, including an animal study that showed the presence of PVC particles in portal bloodstream and lymph vessels after the oral administration of PVC particles (5–110 μm)39. Persorption is the purely mechanical passage of large, solid, undissolved particles as large as approximately 150 μm in diameter from components in the gastrointestinal tract through gaps in the epithelium, known as desquamation zones40,41. Another potential route for the uptake of large MP particles is from iatrogenic sources. Zhu et al. found MP particles in three of five PP-bottled infusion therapy sets, three of five PE-bagged sets, and one of five glass-bottled sets on the market, although their concentration in the products was low42.
Among the total amount of MPs in the environment, it has been estimated that secondary MPs account for 70–80% and primary MPs for 15–30%43. Plastic demand for European countries, including 27 European Union member states, Norway, Switzerland, and the United Kingdom, was 54 Mt in 2022, with 39% used for packaging44. One source of secondary MPs is food packaging, which humans can be directly exposed to via the oral route. An increase in the use of plastic food containers has become a concern concomitant with the detection of MP particles in these containers29. A recent study reported that plastic food containers and reusable food pouches could release secondary MP particles through microwave heating or refrigerated storage30. In our study, the percentage of plastic containers among all containers in the refrigerator was significantly and positively associated with the number of MP in the blood, which is consistent with previous studies that reported the detection of MPs in plastic containers. Similarly, a report that phthalate metabolites were higher among those who used plastics in refrigerator food storage compared to those using other materials also implies that plastic container usage is associated with oral exposure to the breakdown materials of those containers45. Another notable finding is that MPs were detected more in people with higher education levels. In South Korea, people with high incomes prefer bottled water to tap water46, which could contribute to higher exposure to MPs7. This could explain the positive association between education levels and MPs.
Our results show a significant association between MP particles in the blood and higher aPTT and lower antithrombin III levels, implying that the decreased coagulability could be induced by MP particles in the blood. An animal experiment showed that increasing PS MPs in plasmas and increased prothrombin time were significantly correlated, and clotting factors VII, IX, VIII, XI, protein S, and protein C could bind to isolated MPs24. The liver is the major organ responsible for producing plasma clotting factors47,48, and decreased liver function could also be associated with the body burden of MPs15. On the other hand, MPs and elevated platelet count were marginally significantly associated in our study. Wang et al. demonstrated the presence of MPs in human lung tissues from 12 nonsmoking patients and reported that the levels of MPs in lung tissues were positively correlated with platelets and thrombocytosis49. Although it has been studied that zebrafish exposed to PS MPs showed an increase in the expression of genes encoding platelets, which might activate large numbers of platelets50, the detailed mechanism of this association has yet to be known. Finally, the role of MPs in cardiovascular disease has to be interpretated cautiously because of the low numbers and the small number of important factors.
Furthermore, the chemical composition of MPs could affect the balance in the coagulation pathway in diverse ways. The study using human plasma disclosed that the PS nanoparticles could induce thrombin generation, whereas amine-modified nanoparticles could decrease thrombin formation24. Additionally, additives such as phthalates could be another hurdle for coagulation hemostasis. A study of 1,482 pregnant women proved that their urinary phthalate metabolites were positively associated with prolonged blood clotting time as measured by aPTT51. Because blood coagulation is a complex process involving various cells and factors, it may be difficult to assume that MPs act in the same direction on these cells and factors. Future studies are needed to determine whether MPs in human blood are associated with hypercoagulability and to investigate the underlying mechanisms.
A significant and positive correlation between hsCRP and MP counts was found. Although alterations in coagulation were suggested in our study, the plausible mechanistic pathway of MP particles in the blood should be investigated in further studies. Previous research has reported that MPs could cause inflammation, so we found a significant and positive association between hsCRP and MPs. In mice, inflammatory markers, including elevated IL-6 and CRP, were associated with PS52. The significant association was also reported in a human cell-line study53,54. Chronic inflammation could be a decisive driver of atherosclerosis, as insufficient inflammation resolution could promote lipid core formation55. Additionally, it has been reported in an animal study that exposure of hamsters to PS particles was associated with thrombotic events56. These inflammatory responses and alterations in coagulation seem to be a risk factor for cardiovascular disease, although the underlying mechanism has not been fully revealed. A recent study analyzed asymptomatic carotid plaques in 257 patients who underwent carotid endarterectomy and found that 58.4% of patients with MPs in carotid artery plaques had a higher risk of cardiovascular events than those without MPs26. More recently, a study with 82 acute coronary syndrome patients and 19 controls showed that the patient group showed higher MPs in their blood as well as inflammation-related cytokines such as IL-6 and IL-12p70.
This study had several limitations. First, the study samples were not generalizable and were conveniently recruited, even though they were all healthy adults. Second, every endeavor was made to effectively adopt preventive measures for quality control, but laboratory contamination cannot be completely excluded. Third, MPs were primarily detected by the µ-FTIR method, which cannot detect MPs smaller than 5–20 μm. As the size of microplastics detected by Raman was generally smaller than 20 μm, Raman proved to be more advantageous for analyzing smaller particles. However, a Raman analysis requires a significant amount of time to analyze each particle, so it was not possible to apply this method to all samples in this study. Future studies could employ additional methods, such as µ-Raman and Py-GC/MS, to further investigate microplastics. Fourth, the association between the number of MP particles in human blood and coagulation markers was investigated. The total mass in the blood can be obtained using Py-GC/MS, as reported in a study18. For the present study, µ-FTIR and the number of MP particles were applied. However, the most effective and appropriate way to assess MP exposure is yet to be determined. Further studies are necessary to investigate the association between human health indices and MPs measured by various means (Py-GC/MS, µ-FTIR, and µ-Raman).
Conclusion
The current study found that MPs are present in human blood and positively correlate with various lifestyle factors and notable changes in coagulation markers. This emphasizes the need for methods to reduce MP exposure in humans and to further investigate the health effects of MPs, especially with regard to blood coagulation and possible cardiovascular hazards.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
-
PlasticsEurope. Annual production of plastics worldwide from 1950 to 2022 (in million metric tons) [Graph]. [cited 2023 April 23]; (2023). https://www.statista.com/statistics/282732/global-production-of-plastics-since-1950/
-
World Economic Forum. The new plastics economy rethinking the future of plastics. in World Economic Forum. (2016).
-
Thompson, R. C. et al. Lost at sea: where is all the plastic? Science 304 (5672), 838–838 (2004).
Google Scholar
-
Arthur, C., Baker, J. E. & Bamford, H. A. Proceedings of the International Research Workshop on the Occurrence, Effects, and Fate of Microplastic Marine Debris, September 9–11, University of Washington Tacoma, Tacoma, WA, USA. 2009. (2008).
-
Auta, H. S., Emenike, C. U. & Fauziah, S. H. Distribution and importance of microplastics in the marine environment: a review of the sources, fate, effects, and potential solutions. Environ. Int. 102, 165–176 (2017).
Google Scholar
-
Kumar, R. et al. Micro (nano) plastics pollution and human health: how plastics can induce carcinogenesis to humans? Chemosphere 298, 134267 (2022).
Google Scholar
-
Cox, K. D. et al. Human consumption of microplastics. Environ. Sci. Technol. 53 (12), 7068–7074 (2019).
Google Scholar
-
Hernandez, L. M. et al. Plastic Teabags Release Billions of Microparticles and Nanoparticles into tea, vol. 21, 12300–12310 (Environmental Science & Technology, 2019).
-
Li, D. et al. Microplastic release from the degradation of polypropylene feeding bottles during infant formula preparation. Nat. Food. 1 (11), 746–754 (2020).
Google Scholar
-
Kutralam-Muniasamy, G. et al. Consumption of commercially sold dried fish snack Charales contaminated with microplastics in Mexico. Environ. Pollut. 332, 121961 (2023).
Google Scholar
-
Ibrahim, Y. S. et al. Detection of microplastics in human colectomy specimens. JGH Open. 5 (1), 116–121 (2021).
Google Scholar
-
Abbasi, S. & Turner, A. Human exposure to microplastics: a study in Iran. J. Hazard. Mater. 403, 123799 (2021).
Google Scholar
-
Huang, S. et al. Detection and analysis of microplastics in human sputum. Environ. Sci. Technol. 56 (4), 2476–2486 (2022).
Google Scholar
-
Amato-Lourenço, L. F. et al. Presence of airborne microplastics in human lung tissue. J. Hazard. Mater. 416, 126124 (2021).
Google Scholar
-
Horvatits, T. et al. Microplastics Detected in Cirrhotic Liver Tissue. EBioMedicine. 82. (2022).
-
Ragusa, A. et al. Raman microspectroscopy detection and characterisation of microplastics in human breastmilk. Polymers 14 (13), 2700 (2022).
Google Scholar
-
Yan, Z. et al. Analysis of Microplastics in Human Feces Reveals a Correlation between Fecal Microplastics and Inflammatory Bowel Disease Status, vol. 1, 414–421 (Environmental Science & Technology, 2021).
-
Leslie, H. A. et al. Discovery and quantification of plastic particle pollution in human blood. Environ. Int. 163, 107199 (2022).
Google Scholar
-
Guan, Q. et al. The landscape of micron-scale particles including microplastics in human enclosed body fluids. J. Hazard. Mater. 442, 130138 (2023).
Google Scholar
-
Yang, Y. et al. Detection of Various Microplastics in Patients Undergoing Cardiac Surgery, vol. 30, 10911–10918 (Environmental Science & Technology, 2023).
-
Brits, M. et al. Quantitation of micro and nanoplastics in human blood by pyrolysis-gas chromatography–mass spectrometry. Microplast. Nanoplast. 4 (1), 12 (2024).
Google Scholar
-
Yong, C. Q. Y., Valiyaveettil, S. & Tang, B. L. Toxicity of microplastics and nanoplastics in mammalian systems. Int. J. Environ. Res. Public Health. 17 (05), 1509 (2020).
Google Scholar
-
Ali, N. et al. The potential impacts of micro-and-nano plastics on various organ systems in humans. EBioMedicine. 99 (2024).
-
Oslakovic, C. et al. Polystyrene nanoparticles affecting blood coagulation. Nanomed. Nanotechnol. Biol. Med. 8 (6), 981–986 (2012).
Google Scholar
-
Sun, M. et al. Cardiovascular toxicity assessment of polyethylene nanoplastics on developing zebrafish embryos. Chemosphere 282, 131124 (2021).
Google Scholar
-
Marfella, R. et al. Microplastics and nanoplastics in atheromas and cardiovascular events. N. Engl. J. Med. 390 (10), 900–910 (2024).
Google Scholar
-
Johanson, G. A. & Brooks, G. P. Initial scale development: sample size for pilot studies. Educ. Psychol. Meas. 70 (3), 394–400 (2009).
Google Scholar
-
World Health Organization. WHO Guidelines on Physical Activity and Sedentary Behaviour (World Health Organization, 2020).
-
Fadare, O. O. et al. Microplastics from consumer plastic food containers: are we consuming it? Chemosphere 253, 126787 (2020).
Google Scholar
-
Hussain, K. A. et al. Assessing the Release of Microplastics and Nanoplastics from Plastic Containers and Reusable food Pouches: Implications for Human Health 9782–9792 (Environmental Science & Technology, 2023).
-
Vethaak, A. D. & Legler, J. Microplastics and human health. Science 371 (6530), 672–674 (2021).
Google Scholar
-
Primpke, S. et al. An automated approach for microplastics analysis using focal plane array (FPA) FTIR microscopy and image analysis. Anal. Methods. 9 (9), 1499–1511 (2017).
Google Scholar
-
Leonard, V. L. Microplastics in human blood: polymer types, concentrations and characterisation using µFTIR. Environ. Int. 188, 108751 (2024).
Google Scholar
-
Kutralam-Muniasamy, G. et al. Microplastic diagnostics in humans:the 3Ps Progress, problems, and prospects. Sci. Total Environ. 856, 159164 (2023).
Google Scholar
-
Deng, Y. et al. Tissue accumulation of microplastics in mice and biomarker responses suggest widespread health risks of exposure. Sci. Rep. 7 (1), 46687 (2017).
Google Scholar
-
Sun, W. et al. Blood uptake and urine excretion of nano- and micro-plastics after a single exposure. Sci. Total Environ. 848, 157639 (2022).
Google Scholar
-
Hussain, N., Jaitley, V. & Florence, A. T. Recent advances in the understanding of uptake of microparticulates across the gastrointestinal lymphatics. Adv. Drug Deliv. Rev. 50 (1), 107–142 (2001).
Google Scholar
-
Rotchell, J. M. et al. Detection of microplastics in human saphenous vein tissue using µFTIR: a pilot study. PLoS One. 18 (2), e0280594 (2023).
Google Scholar
-
Volkheimer, G. Hematogenous dissemination of ingested polyvinyl chloride particles. Ann. N. Y. Acad. Sci. 246, 164–171 (1975).
Google Scholar
-
Gardner, M. L. & Steffens, K. J. and K.-J. Steffens. Persorption—criticism and agreement as based upon in vitro and in vivo studies on mammals. In Absorption of Orally Administered Enzymes (Springer, 1995).
-
Wright, S. L. & Kelly, F. J. Plastic and Human Health: A micro Issue? 6634–6647 (Environmental Science & Technology, 2017).
-
Zhu, L. et al. Microplastics entry into the blood by infusion therapy: few but a direct pathway. Environ. Sci. Technol. Lett. 11 (2), 67–72 (2023).
Google Scholar
-
Mariano, S. et al. Micro and nanoplastics identification: classic methods and innovative detection techniques. Front. Toxicol. 3, 636640 (2021).
Google Scholar
-
Plastics Europe. Plastics—the fast Facts 2023. https://plasticseurope.org/knowledge-hub/plastics-the-fast-facts-2023/ (2023).
-
Kang, J., Cho, S. & Yoon, S. Relationship between the use of Plastics in Refrigerator food Storage and Urine Phthalate Metabolites: The Korean National Environmental Health Survey (KoNEHS) Cycle 3 35 (Annals of Occupational and Environmental Medicine, 2023).
-
Kim, Y. S. A path analysis investigation of risk perception and satisfaction of Tap Water. J. Korean Home Manag. Assoc. 9 (3), 1–22 (2006).
Google Scholar
-
Emeis, J. et al. A guide to murine coagulation factor structure, function, assays, and genetic alterations. J. Thromb. Haemost. 5 (4), 670–679 (2007).
Google Scholar
-
Thachil, J. Relevance of clotting tests in liver disease. Postgrad. Med. J. 84 (990), 177–181 (2008).
Google Scholar
-
Wang, S. et al. Microplastics in the lung tissues associated with blood test index. Toxics. 11(9). (2023).
-
Gao, N. et al. Impact and molecular mechanism of microplastics on zebrafish in the presence and absence of copper nanoparticles. Front. Mar. Sci. 8, 762530 (2021).
Google Scholar
-
Jiang, M. et al. Urinary concentrations of phthalate metabolites associated with changes in clinical hemostatic and hematologic parameters in pregnant women. Environ. Int. 120, 34–42 (2018).
Google Scholar
-
Dingjie, H. et al. Polystyrene microplastic exposure induces insulin resistance in mice via dysbacteriosis and pro-inflammation. Sci. Total Environ. 838, 155937 (2022).
Google Scholar
-
Busch, M. et al. Investigations of acute effects of polystyrene and polyvinyl chloride micro-and nanoplastics in an advanced in vitro triple culture model of the healthy and inflamed intestine. Environ. Res. 193, 110536 (2021).
Google Scholar
-
KC, P. B. et al. Polytetrafluorethylene microplastic particles mediated oxidative stress, inflammation, and intracellular signaling pathway alteration in human derived cell lines. Sci. Total Environ. 897, 165295 (2023).
Google Scholar
-
Bäck, M. et al. Inflammation and its resolution in atherosclerosis: mediators and therapeutic opportunities. Nat. Rev. Cardiol. 16 (7), 389–406 (2019).
Google Scholar
-
Nemmar, A. et al. Ultrafine particles affect experimental thrombosis in an in vivo hamster model. Am. J. Respir. Crit. Care Med. 166 (7), 998–1004 (2002).
Google Scholar
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) Funded by the Ministry of Education (RS-2023-00248956).
Author information
Authors and Affiliations
Contributions
D.W.-L.: conceptualization, methodology, investigation, data curation, writing—original draft, visualization; J.-J.: methdology, investigation, writing-original draft, visualization; S.-P: investigation, writing—review and editing; Y.-L.: investigation, writing—review and editing; J.-K.: investigation, writing—review and editing; C.-H.: validation, writing—review and editing; H.C.-K.: validation, writing—review and editing; J.H.-L.: validation, writing—review and editing; Y.C.-H., supervison, writing-review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
Reprints and permissions
About this article
Cite this article
Lee, DW., Jung, J., Park, Sa. et al. Microplastic particles in human blood and their association with coagulation markers.
Sci Rep 14, 30419 (2024). https://doi.org/10.1038/s41598-024-81931-9
-
Received: 19 August 2024
-
Accepted: 30 November 2024
-
Published: 06 December 2024
-
DOI: https://doi.org/10.1038/s41598-024-81931-9
Keywords
- Microplastics
- Human whole blood
- Blood coagulation
- Fourier-transform infrared spectroscopy
- Inflammation markers
- Lifestyle factors