Abstract
Head and neck cancer (HNC) is the seventh most common cancer globally, with 20–60% of patients experiencing nutritional deficiencies. Recent studies indicate that microRNAs (miRNAs) may serve as molecular markers for malnutrition. This study evaluated miR-22-3p as a potential predictor of nutritional deficiencies and a prognostic factor in HNC patients undergoing intensity-modulated radiation therapy (IMRT). From 2014 to 2017, fifty-six advanced HNC patients at the Medical University of Lublin received IMRT, with miR-22-3p levels measured from peripheral blood before treatment. Statistical analysis using MedCalc 15.8 revealed that underweight patients had significantly lower miR-22-3p expression compared to non-underweight patients (0.89 vs. 2.47; p = 0.0233). Moderately or severely malnourished patients also showed reduced miR-22-3p levels compared to well-nourished individuals (1.42 vs. 11.04; p = 0.026). Additionally, patients with critical weight loss (CWL) had significantly lower miR-22-3p levels than those without CWL (0.96 vs. 4.91; p = 0.0015). Weak correlations were found between miR-22-3p levels, cancer stage, body mass index (BMI), and C-reactive protein (CRP), with lower miR-22-3p levels linked to advanced tumor stages and higher CRP levels. This study suggests miR-22-3p as a biomarker for nutritional deficiency risk in HNC patients, though further research is needed to validate its predictive capacity.
Introduction
Head and neck cancer (HNC) ranks as the seventh most prevalent cancer globally, with an estimated 0.66–1.1 million new cases annually, contributing to approximately 325,000-500,000 annual deaths1,2. HNC encompasses a diverse array of tumors affecting the upper respiratory and upper gastrointestinal tracts1. In addition to smoking, acknowledged as the primary risk factor for HNC3, others identified encompass: regular high alcohol consumption, genetic predisposition, laryngopharyngeal reflux, areca (betel) nut chewing, marijuana usage, inadequate oral hygiene and prior exposure to radiotherapy (RT)4,5. Squamous cell carcinoma (SCC) originating in the lining of the mouth, pharynx, and larynx, accounts for up to 90% of HNC cases6. Currently, also infection agents i.e. Human Papillomavirus (HPV), especially HPV16/18 accounting for nearly 90% of HPV-related oropharyngeal squamous cell carcinoma (OSCC) and Epstein-Barr virus (EBV) linked particularly with nasopharyngeal cancer (NPC) are recognized as significant factors involved in HNC etiopathogenesis7,8. As per the National Comprehensive Cancer Network (NCCN) Guidelines, about 30–40% of patients with early-stage cancer (stage I or II) are typically recommended for single-modality treatment, either surgery or RT. Surgery is often the preferred choice for oral cavity and paranasal sinus cancers, while RT, with or without chemotherapy (CTH), is predominantly recommended for all stages of NPC. For approximately 60% of patients diagnosed with locally or regionally advanced disease, combined modality therapy is advised. When administering CTH alongside radiation, cisplatin is typically the preferred radiosensitizer9. In the primary treatment setting, RT is commonly administered in a fractionated regimen, culminating in a cumulative total dose ranging from 66 to 74 Gy. Postoperative RT typically involves a lower cumulative dose (50–66 Gy). In high-risk situations, the standard nonsurgical approach involves combining RT with concurrent CTH. Other agents used concurrently include cetuximab, a monoclonal antibody targeting the epidermal growth factor receptor (EGFR), and avelumab, a Programmed Death-Ligand 1 (PDL-1) inhibitor10,11. Recent phase III Randomized Controlled Trials (RCTs) have demonstrated the efficacy of immunotherapy in patients with recurrent, unresectable, or metastatic HNC9.
In the case of HNC patients, malnutrition is prevalent in 20–60% of cases, stemming from various factors12,13. These include metabolic disorders associated with cancer and compromised swallowing function due to tumor location, significantly hindering oral food intake; additionally, the side effects of treatments (such as surgery, RT, chemoradiotherapy (CRT), or multimodal treatments) can affect nutritional status14,15.
Nutritional deficiencies significantly impact cancer patients, leading to prolonged hospital stays and poor treatment outcomes16. The variability in percentages for malnutrition or cachexia significantly depends on the chosen criteria, as a single definition was not established until the European Society for Clinical Nutrition and Metabolism (ESPEN) consensus in 2015. However, not only before but also after the introduction of consensus in various studies different criteria were adopted, which commonly resulted in obtaining incomparable data and probably led to an underestimation of nutritional deficiencies occurrence in many cases13,15. As per ESPEN criteria, the criteria for being at nutritional risk must be met before diagnosing malnutrition. Diagnostic confirmation includes having a reduced body mass index (BMI) < 18.5 kg/m² according to the World Health Organization’s (WHO) underweight definition, or combined weight loss (WL) and reduced BMI based on age-dependent cut-offs, or reduced gender-dependent fat-free mass index (FFMI)17. Malnutrition, classified into disease-related and non-disease related, is assessed through the Global Leadership Initiative on Malnutrition (GLIM) criteria, established in 2016. These criteria outline a three-stage diagnosis process. The initial stage employs validated questionnaires for screening, followed by confirmation using phenotypic criteria such as WL, low BMI, and reduced muscle mass, along with etiological factors like diminished food intake or disease burden. The severity of malnutrition is determined in the third stage, categorizing it based on phenotypic criteria. GLIM criteria differentiate malnutrition types including chronic disease-related with or without inflammation, acute disease-related with severe inflammation, and starvation due to socio-economic or environmental factors18. Cancer cachexia is a complex syndrome involving metabolic changes across tissues, leading to skeletal muscle and adipose tissue loss alongside inflammation19. In HNC, understanding molecular mechanisms of this syndrome is vital. Muscle hypertrophy relies on the insulin-like growth factor-1/Phosphoinositide 3-kinase/Protein Kinase B (IGF-1/PI3K/AKT) pathway, affected by genetic variations in genes encoding IGFs20. AKT plays a key role in cellular survival and metabolism, interacting with Nuclear Factor kappa B (NFκB) and B-cell lymphoma 2 (Bcl-2) proteins21. Muscle atrophy involves the activation of ubiquitin ligases like Muscle RING Finger 1(MuRF1)/atrogin-1, driven by Forkhead Box O (FOXO) isoforms20. Additionally, the tumor necrosis factor receptor (TNFR) associated factor 6 (TRAF6) gene participates in cytokine-induced pathways22. Peroxisome proliferator-activated receptors (PPARs), particularly PPARα and PPARγ, regulate lipid metabolism and inflammation23. The acute phase response (APR) increases liver synthesis of defense proteins (like C-reactive protein (CRP)), but can worsen muscle wasting in cancer patients, potentially predicting adverse outcomes24. Polymorphisms in cytokine genes like tumor necrosis factor alfa (TNF-α), IL-1β, IL-6, and IL-18 are associated with altered transcription, which plays a role in the development of cachexia and poorer clinical outcomes25.
To date, many studies have examined the utility of various markers, including the albumin, prealbumin, transferrin26, CRP, IL-6, IL-8, IL-18, osteoprotegerin (OPG), and soluble-Receptor-For-TNF-alpha sTNFRII27,28, as well as neutrophil-to-lymphocyte ratio (NLR)28,29 in the assessment of the nutritional status of patients. ESPEN guidelines propose measuring CRP as a potential biomarker of malnutrition. However despite that, currently in clinical practice, there are no established biomarkers for predicting or screening malnutrition (including HNC)17,18. GLIM criteria, in addition to serum CRP propose also albumin, or pre-albumin as useful indicators18. Recent studies have suggested the potential utility of various microRNAs (miRNAs) (miR-130a, miR-21, miR-378 and miR-511-3p) as molecular markers for malnutrition30,31,32,33,34. miRNAs are short (20–24 nt) single-stranded noncoding RNAs that can be detected in cells and body fluids35. miRNAs oversee gene expression post-transcriptionally and play a pivotal role in regulating pathways linked to nutrient metabolism and inflammation35,36.
The human genome location for miR-22 is 17p13.3, the length of immature and mature miR-22 is approximately 85 and 22 nucleotides, respectively37,38. To date, miR-22-3p has been studied in many cancers including gastric cancer (GC), colorectal cancer (CRC), papillary thyroid carcinoma (PTC), however, research were focused solely on the role of this molecule in the pathogenesis, early detection and treatment of these cancers39,40,41,42.
On the other hand, miR-22-3p is implicated in diverse metabolic processes and inflammatory responses, and a multitude of genes affecting nutritional status exhibit correlations with miR-22-3p. Notable instances encompass genes that encode proteins within the AKT serine-threonine kinase family, specifically AKT1 and AKT3. These kinases play pivotal roles in governing metabolism, proliferation, angiogenesis, apoptosis, glycogen synthesis, and glucose uptake. Furthermore, they modulate cellular signaling cascades in response to insulin and growth factors43,44. Another gene associated with miR-22-3p is CPT1A, responsible for encoding carnitine palmitoyltransferase 1 A, an enzyme that plays a crucial role in the beta-oxidation of fatty acids (FA) within mitochondria45. ADAM17, a gene implicated in the initiation of inflammation and concurrently linked to miR-22-3p, encodes a membrane protein referred to as TNFα-Converting Enzyme (TACE). This enzyme’s capability to cleave TNFα results in the release of its soluble form, thereby inducing inflammation46. Furthermore, miR-22-3p is implicated in malnutrition in HNC patients due to its role in skeletal muscle fiber-type conversion by inhibiting the AMPK/SIRT1/PGC-1α signaling pathway47. It also affects the proliferation and differentiation of skeletal muscle cells by targeting IGFBP348 and HDAC449 and promotes intestinal cells proliferation by targeting CCAAT/enhancer-binding protein δ (C/EBPδ)50. Additionally, its upregulation is associated with decreased fibro/adipogenic progenitor (FAP) adipogenesis and reduced fatty degeneration in muscles via the miR-22-3p/KLF6/MMP-14 pathway51. Notably, decreased levels of miR-22-3p lead to the up-regulation of TCF7, impairing gluconeogenesis by inhibiting the expression of gluconeogenesis enzymes52.
Considering the above and the fact, that to date, no studies assess the relationship between the expression of this miRNA and the occurrence of nutritional deficiencies in patients with HNC or any other cancer or other disease this cross-sectional study aimed to assess the miR-22-3p as a potential predictor of nutritional deficiencies and prognostic factor in patients with HNC subjected to intensity-modulated radiation therapy (IMRT).
Results
Characteristic of study group
The study group was predominantly male (83.9%). The median age of the patients was 62.5 years, ranging from 42 to 87 years. Histopathological examination confirmed the presence of Head and Neck Squamous Cell Carcinoma (HNSCC) in all patients. Patients were at stage III (28.6%) or IV (71.4%) according to the tumor, node, and metastasis clinical staging system (TNM).
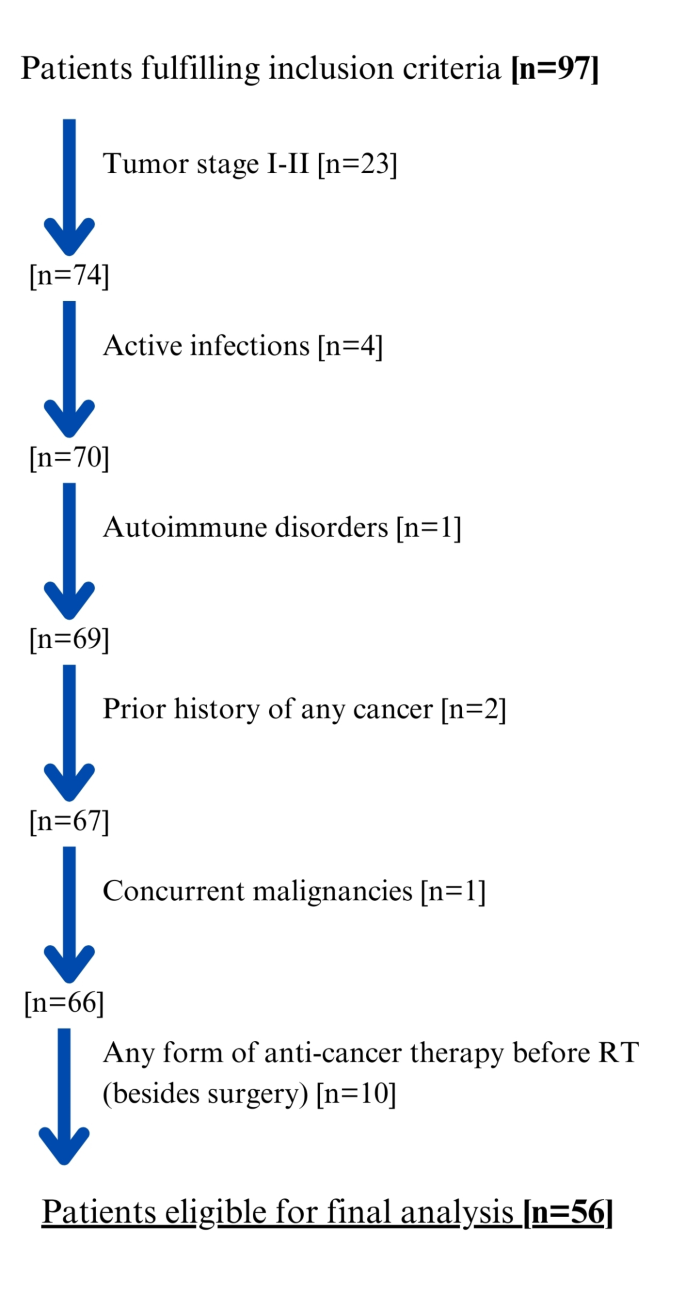
The Patient Flow Chart is presented in Fig. 1. Regarding tumor location, 10.7% of patients had tumors in the nasopharynx (n = 6), 17.8% in the oropharynx (n = 10), 1.8% in the hypopharynx (n = 1), and 3.6% in the oral cavity (n = 2), while 60.7% had tumors the laryngeal area. In 5.4% of cases (n = 3), tumors were located in other sites, such as the palatine tonsil, paranasal sinus, or maxillary sinus. Surgical treatment was conducted in 73.2% of the patients, while combined CRT in 32.1% and RT in 51.8%. RT without prior surgery was used in 16.1% of patients (Detailed information on treatment depending on the location of the tumor is presented in Table S1, Supplementary Materials). Among those who underwent surgery, 90.2% had tumor resection extended by lymphadenectomy. On average, the time between surgery and nutritional assessment was 71 days.

Patient flow Chart. RT radiotherapy.
In the preoperative period none of the patients received any nutritional support. In the post-operative period, nutritional support included the use of Percutaneous Endoscopic Gastrostomy (PEG) in 3.6% of cases and feeding tubes in 35.7% of patients. On average, nutritional support in the post-operative period was administered for 15.5 days. During RT, oral nutrition support was utilized in 3.6% of patients, while parenteral nutrition was employed in 10.7% of patients. None of the patients received nutritional support during post-RT period. Detailed characteristics of the patients are provided in Table 1.
Nutritional assessment
In our study, 30.4% of patients had a high risk of malnutrition (NRS ≥ 3) according to the NRS 2002 scale. Additionally, according to the Patient-Generated Subjective Global Assessment (SGA), 46.4% of patients were classified as moderately malnourished (B), while 33.9% were categorized as severely malnourished (C). Furthermore, 30.4% of the patients were diagnosed with CWL, as indicated in Table 1.
Mir-22-3p expression in predicting the occurrence of nutritional deficiencies
In our study, patients classified as underweight based on BMI displayed notably diminished miR-22-3p expression compared to those without underweight (0.89 vs. 2.47; p = 0.0233; Fig. 2A). Similarly, patients identified with moderate or severe malnutrition via SGA exhibited significantly reduced expression of the studied miRNA in contrast to well-nourished individuals (1.42 vs. 11.04; p = 0.026; Fig. 2B). Moreover, those with severe malnutrition displayed notably lower miR-22-3p expression compared to well-nourished or moderately malnourished patients (0.98 vs. 4.95; p = 0.0049; Fig. 2C). Additionally, patients experiencing CWL demonstrated significantly decreased expression of miR-22-3p compared to those without CWL (0.96 vs. 4.91; p = 0.0015; Fig. 2D).

Relative expression of miR-22-3p depending on body weight status according to BMI (WHO) (A); nutritional status (according to SGA) (B,C); critical weight loss occurrence (D). BMI Body Mass Index, SGA-Patient-Generated subjective global assessment, WHO World Health Organization.
The analysis of miR-22-3p expression revealed 100% sensitivity and 41.3% specificity in predicting underweight based on BMI (AUC = 0.73; p = 0.0054; Fig. 3A). Conversely, miR-22-3p showed 77.8% sensitivity and 63.6% specificity in predicting moderate or severe malnutrition (AUC = 0.72; p = 0.0187; Fig. 3B). Notably, high miR-22-3p levels enabled the prediction of severe malnutrition with 89.5% sensitivity and 59.5% specificity (AUC = 0.73; p = 0.0008; Fig. 3C). Prediction of CWL through miR-22-3p expression analysis demonstrated 88.2% sensitivity and 64.1% specificity (AUC = 0.77; p = 0.0002; Fig. 3D).

The assessment of the relative expression of miR-22-3p in predicting the occurrence of nutritional deficiencies conducted using ROC analysis: Underweight according to BMI (WHO) (A); Moderate or severe malnutrition (according to SGA) (B); severe malnutrition (according to SGA) (C); critical weight loss (D). BMI body mass index, AUC area under the curve, CWL critical weight loss, SGA patient-generated subjective global assessment, ROC receiver operating characteristic, WHO World Health Organization.
Patients exhibiting high miR-22-3p expression faced significantly lower risks—more than 5.5-fold—of developing moderate or severe malnutrition according to SGA (OR = 0.18; p = 0.0185). High miR-22-3p expression correlated with nearly an 8-fold decrease in the risk of severe malnutrition according to SGA (OR = 0.13; p = 0.0039). Furthermore, individuals with high miR-22-3p expression exhibited over a 14-fold lower risk of CWL (OR = 0.07; p = 0.0016).
Multivariable analysis highlighted that patients with high miR-22-3p expression faced significantly reduced risks—over 5.5-fold—of developing moderate or severe malnutrition according to SGA (OR = 0.18; p = 0.0257). Similarly, high miR-22-3p expression was associated with nearly a 5-fold decrease in the risk of severe malnutrition according to SGA scale (OR = 0.21; p = 0.0393). Additionally, high levels of miR-22-3p correlated with a 25-fold lower risk of CWL (OR = 0.04; p = 0.0018). Detailed data, including comparisons of miR-22-3p expression based on nutritional status, the diagnostic utility of miR-22-3p expression in predicting nutritional deficiencies, and risk assessments based on miR-22-3p expression levels, are presented in Tables 2 and 3.
Correlation between mir-22-3p expression and nutritional status indicators
A statistically significant weak, negative correlation emerged between the N feature (rho = -0.267; p = 0.0465) or M feature (rho= -0.267; p = 0.0310) and miR-22-3p expression level. Similarly, a statistically significant weak, negative correlation was observed between the cancer stage and the expression of the examined miRNA (rho = -0.345; p = 0.0092). Conversely, a weak but statistically significant positive correlation was found between BMI value and miR-22-3p levels (rho = 0.348; p = 0.0086; Fig. 4A). Furthermore, a moderate, negative correlation was identified between malnutrition as per the SGA scale and miR-22-3p expression (rho = -0.418; p = 0.0014). Another weak, negative correlation was noted between CRP concentration and miR-22-3p expression (rho = -0.399; p = 0.0023; Fig. 4B). Comprehensive data are provided in Table 4.

Correlation between the relative expression of miR-22-3p and BMI (A) or CRP (B). BMI body mass index, CRP C-reactive protein.
Overall survival
The findings regarding the impact of demographic, clinical, nutritional, and epigenetic variables on overall survival (OS) are outlined in Table 5.
Univariable analysis
Patients with the M1 feature, those in the stage IVC of the cancer (in both cases: approximately 60-fold; HR = 59.31; p = 0.0001), and those requiring parenteral nutrition (over 5-fold; HR = 5.32; p = 0.0453) faced a significantly elevated risk of death.
Multivariable analysis
Only patients with the M1 feature and in the stage IV of the cancer exhibited a significantly higher risk of death, exceeding 6-fold (in both cases: HR = 6.4; p = 0.0005).
Comparison of mir-2-3p relative expression depending on demographic, clinical and nutritional variables
A significantly lower expression of miR-22-3p was observed in patients at the T4 tumor stage compared to T1-T3 stages. Moreover, M1 stage and the stage IVC of the cancer (according to TNM classification) were related to a lower expression of the studied miRNA. Expression of miR-22-3p was significantly higher in patients requiring nutritional support during RT. Additionally, patients with high CRP level had significantly lower expression of studied miRNA (Table S2, Supplementary Materials).
Factors affecting the underweight
The results of the influence of demographic, clinical, and nutritional variables on underweight are presented in Table S3 (Supplementary Materials).
Univariable analysis
Patients with higher degree (< 3) of nutritional risk according to the NRS scale had significantly higher risk (more than 8- fold) of underweight according to BMI (WHO) (OR = 8.40; p = 0.0062). Moreover, patients with elevated CRP concentrations had significantly higher risk (7.5-fold) of underweight according to BMI (OR = 7.5; p = 0.0176).
Multivariable analysis
Patients with higher degree (< 3) of nutritional risk according to the NRS scale had significantly higher risk (more than 16- fold) of underweight according to BMI (WHO) (OR = 16.11; p = 0.0042). Moreover, patients with elevated CRP concentrations had significantly higher risk (nearly 15-fold) of underweight according to BMI (WHO) (OR = 14.78; p = 0.0096).
Factors affecting the risk of Higher Nutritional Risk according to the NRS Scale
None of the studied variables was significantly associated with nutritional risk according to the NRS scale (Table S4, Supplementary Materials).
Factors affecting the risk of malnutrition according to the SGA scale
The results of the influence of demographic, clinical, and nutritional variables on the risk of malnutrition according to SGA are presented in Table S5 (Supplementary Materials).
Univariable analysis
In patients at the T4 tumor stage, the risk of moderate (B) or severe (C) malnutrition according to the SGA scale was nearly 32-fold higher (OR = 31.36; p = 0.0196). In turn, patients at the T4 tumor stage showed significantly, nearly 6-fold higher risk of severe malnutrition (OR = 5.83; p = 0.0050). In the case of smokers, the risk of moderate and severe malnutrition was nearly 5-fold higher (OR = 4.80; p = 0.0274). Higher CRP concentration was associated with nearly 27-fold higher risk of moderate or severe malnutrition (OR = 26.21; p = 0.0268). Moreover, patients with higher CRP levels had a higher risk (over 7.5-fold) of severe malnutrition (OR = 7.56; p = 0.0016).
Multivariable analysis
Smokers had significantly higher (nearly-5 fold) risk of moderate or severe malnutrition (OR = 4.82; p = 0.0397). Patients with T4 tumor stage, the risk of severe malnutrition according to the SGA scale was nearly 5-fold higher (OR = 4.53; p = 0.0257). Higher CRP level was significantly associated with approximately 8- fold higher risk of severe malnutrition (OR = 7.56; p = 0.0016).
Factors affecting the risk of CWL
The outcomes detailing the impact of demographic, clinical, and nutritional variables on the occurrence of CWL are presented in Table S6 (Supplementary Materials).
Univariable analysis
The tumor localization in the nasopharynx was associated with a more than 7-fold higher risk of CWL (OR = 7.10; p = 0.0023). Conversely, tumor localization in the larynx was linked to more than a 7-fold lower risk of CWL (OR = 0.14; p = 0.0027).
Multivariable analysis
Localization of the tumor in nasopharynx was associated with nearly 13-fold higher risk of developing CWL (OR = 12.68; p = 0.0037).
Discussion
In our study, patients classified as underweight (based on BMI) and those with moderate to severe malnutrition showed significantly reduced expression of miR-22-3p compared to well-nourished individuals. Severe malnutrition and CWL were associated with notably lower miR-22-3p expression compared to well-nourished or moderately malnourished patients. MiR-22-3p showed varying yet significant sensitivity and specificity in predicting underweight, malnutrition severity, and CWL. High miR-22-3p expression was linked to significantly lower risks of developing malnutrition or experiencing CWL. Weak correlations were observed between miR-22-3p expression and certain factors: negative correlations with cancer stage, N status, and CRP concentration, and a positive correlation with BMI. Lower miR-22-3p expression was found in advanced tumor stages and disease progression (stage IV according to TNM classification). Patients requiring nutritional support during RT and those with high CRP levels had significantly lower expression of miR-22-3p. Overall, miR-22-3p expression levels demonstrated associations with nutritional status, disease progression, and certain clinical parameters in the studied patient population.
We opted to investigate miR-22-3p as a potential predictor of malnutrition in HNC patients due to its involvement in various physiological processes, including skeletal muscle fiber-type conversion, proliferation, and differentiation of skeletal muscle cells and intestinal cells, as well as its impact on adipogenesis and gluconeogenesis47,48,49,50,51,52. Emphasizing that to date, there hasn’t been a direct assessment regarding the correlation between miR-22-3p and the onset of nutritional disorders. Evaluation, encompassing both animal models and cohort studies, remains absent across diseases linked to such disorders, notably including cancer, particularly HNC. Nevertheless, numerous studies underscore the pivotal role of this miRNA in various carcinogenic processes, thereby highlighting its potential in cancer diagnosis as detection, predictive, or prognostic markers39,40,41,42.
Wang et al. demonstrated a correlation between decreased miR-22-3p levels in CRC and low levels of human bone marrow-derived mesenchymal stem cells (hBMSCs) Ecomes39. Guan et al. showed a link between low miR-22-3p levels in GC and increased expression of noncatalytic region of tyrosine kinase adaptor protein 1 (NCK1-AS1), which exerts carcinogenic effects40. Additionally, Wang et al. and Guo et al. indicated that miR-22-3p levels decreased in PTC due to circ-ITCH overexpression41,42.
Additionally, several studies have highlighted various miRNAs as potential markers for nutritional disorders53,54,55.
Lee et al. conducted a study using animal models, specifically fourteen 8-week-old C57BL/6L mice. Among them, eight mice received injections of Lewis lung carcinoma (LLC), while six received phosphate-buffered saline. The researchers observed significant differences in the expression of nine miRNAs (including increased levels of miR-147-3p, miR-511-3p, miR-223-3p, miR-205-5p, and decreased levels of miR-299a-3p, miR-1933-3p, miR-3473d, miR-431-5p, miR-665-3p) in the muscles of the mice euthanized four weeks after tumor growth. Although the total body weight of the LLC mice was approximately 10% higher than that of the PBS (phosphate-buffered saline) control group, when the tumor weight was subtracted, there was no significant difference in body weight between the groups. However, the LLC mice exhibited significantly smaller tibialis anterior (18.5%) and gastrocnemius (8.6%) muscles compared to the control group. Additionally, the LLC mice had significantly lower epididymal fat mass (approximately 20%) compared to the PBS group. Notably, the expression of miR-511-3p and miR-205-5p was notably higher in the LLC group compared to the PBS group, showing a 1.53-fold change and 2.59-fold change, respectively. These findings suggest a potential correlation between specific miRNAs and cachectic muscle atrophy53.
Takahashi et al. investigated protein malnutrition’s impact on rats, assessing miR-203 expression levels. Male Wistar rats aged 6 weeks comprised the study group. The control (C) group consumed a 20% casein diet (AIN-93G), while the low-protein (LP) group had ad libitum access to a 5% casein diet. After 14 days, the animals were euthanized. The study delineated the hepatic miRNA profile in response to protein malnutrition, revealing that a low-protein diet upregulated Hadhb expression by downregulating miR-203. This effect induced β-oxidation of fatty acids and suggested a potential link between miR-203 and lipid metabolism changes stemming from protein malnutrition. The research suggested that protein intake modulates biological processes via miRNA functionality, indicating the potential use of downregulated miR-203 as a malnutrition biomarker. However, further studies are necessary to validate these findings54.
Qiu et al. investigated the influence of oral SqCC exosomes containing miR-181a-3p on muscle cell atrophy and apoptosis. The study involved 9 female C3H/HeOuJ mice (10 weeks old) in which a cancer cachexia model was developed. Exosomes from oral SqCC cells containing miR-181a-3p were observed to affect the ERS pathway, leading to muscle cell atrophy and apoptosis. To explore its impact on TERS signaling to C2C12 myotubes, both a miR-181a-3p inhibitor and mimic were employed. Inhibiting miR-181a-3p resulted in a slight increase in Grp78 expression, impeding TCM-induced ERS. Conversely, raising miR-181a-3p levels reduced Grp78 gene expression while augmenting other genes and proteins involved in the ERS, muscle atrophy, and apoptosis pathways. These findings suggest miR-181a-3p as a potential target for mitigating muscle atrophy in cancer cachexia patients55.
Although there are no studies on miRNA-22-3p as a potential predictor of malnutrition in HNC, an experimental study on miRNA expression was conducted in hepatocellular carcinoma cells (HCC). Li et al. investigated miRNA-22-3p expression in HCC using normal (HL-7702 cells) and cancerous (QGY-7703 cells) human hepatocytes cultured in modified RPMI 1640 with FA deficiency for 21 days (FA deficiency induces oxidative stress and apoptosis in the well-differentiated HCC cell line HepG256. The study detected changes in miR-22-3p/miR-149-5p expression in response to FA deficiency using Poly (A) Tailing RT-qPCR. The findings indicated that FA deficiency upregulated miR-22-3p/miR-149-5p expression in QGY-7703/HL-7702 cells. This research suggests that miR-22-3p might serve as a potential tumor suppressor in HCC and could act as a biomarker for assessing FA deficiency, potentially reflecting malnutrition in HCC patients57.
Several other studies have explored the potential of miRNAs as predictors of nutritional deficiencies in CRC or HNC34,36,58.
Okugawa et al. conducted a study involving 167 CRC patients who underwent primary resection, evaluating serum miR-21 expression levels in correlation with Psoas Muscle Index (PMI). They observed significantly higher serum miR-21 levels in patients with low PMI compared to those with high PMI, indicating an intimate association between serum miR-21 and skeletal muscle mass. Postoperative reductions in serum miR-21 levels were observed in patients with potentially curative surgeries (Stages I–III), but not in patients with non-curative resections (Stage IV). The study highlighted serum miR-21 as a potential biomarker for monitoring cancer cachexia in CRC patients and as a disease-specific biomarker for CRC prognosis58.
Powrózek et al. investigated the correlation between miR-130a expression and the risk of developing malnutrition in 70 HNC patients undergoing RT. The study evaluated the accuracy of SGA and miRNA in assessing malnutrition or cachexia. Patients with low miRNA-130a expression were found to be more susceptible to cachexia, potentially through the promotion of an inflammatory response mediated by TNF-α. The research suggested miR-130a as a promising tool for predicting cachexia before therapy initiation in HNC patients36.
Mazurek et al. research aimed to assess the change in miR-511-3p expression in a group of patients with HNC. The study group consisted of 60 patients who received treatment consisting of IMRT alone or combined with other treatment methods. The study aimed to evaluate the relationship between the expression of miR-511-3p before treatment and the nutritional status within this particular group. The analysis showed that lower level of miR-511-3p expression was associated with significantly higher risk of moderate or severe malnutrition according to SGA. Also in patients at the T4 tumor stage, the expression of miR-511-3p was lower than in less advanced stages of cancer (according to TNM). There was no significant relation between miR-511-3p expression and nutritional support in the post-operational period and during RT. These results lead to the conclusion that the level of miR-511-3p might be used as a predictor of malnutrition in HNC in the pre-operative period34.
Ferreira et al. conducted a study with the objective of exploring the connections between dietary patterns and the levels of miR-31 and miR-375 expression in individuals recently diagnosed with HNSCC. The study group consisted of 67 patients aged 20 to 80 years at the time of diagnosis who had not yet received any treatment. Dietary intake was assessed using a semi-quantitative food frequency questionnaire (FFQ). They found that higher intake of iron and vitamin C, along with lower intake of total sugar, cholesterol, vitamin B9, and zinc, was associated with higher miR-31 expression. Similarly, elevated consumption of selenium, vitamin C, and vitamin D, coupled with lower intake of added sugar, phosphorus, and vitamin B12, was correlated with higher miR-375 expression. These results underscore significant relationships between dietary habits and the expression of miR-31 and miR-375 in HNSCC patients, suggesting potential avenues for future research into the influence of nutrients on carcinogenesis and the utilization of studied miRNAs in evaluating the nutritional status of patients with HNSCC59.
The study demonstrated that miR-22-3p levels correlate with nutritional status as measured by BMI, SGA, and CWL, but not with the NRS-2002 score. This discrepancy likely arises from the differing focuses of these assessment tools. BMI, SGA, and CWL directly measure nutritional and metabolic status, which are closely tied to the biological processes that miR-22-3p influences, such as inflammation and muscle metabolism43,44,45,46,47,48,49,60. In contrast, NRS-2002, being a broader screening tool that incorporates subjective assessments of disease severity and nutritional intake, may dilute the specific metabolic signals that miR-22-3p captures61. Consequently, while miR-22-3p effectively reflects nutritional deficiencies linked to metabolic changes, its lack of correlation with NRS-2002 suggests that NRS-2002 might be less effective in detecting the specific biological alterations associated with miR-22-3p expression.
Our work presents a range of miR-22 cut-off points that may be associated with different types of nutritional deficiencies. Depending on the tools used to assess this type of disorder in a given medical facility, clinicians can select the appropriate miR-22 cut-off point that will represent a specific nutritional deficiency. According to our analysis, miR-22 values in the range of 2.93–5.28 may be associated with the risk of moderate to severe malnutrition, while values in the range of 1.92–2.92 and lower may indicate severe malnutrition or even critical weight loss. Since detecting patients at risk of developing nutritional disorders (at the earliest possible stage of development of such disorders) is crucial for their proper care, based on the analysis, it can be suggested that the miR-22 level that should arouse clinical alert is approximately 5.28.
Compared to traditional parameters like BMI, SGA, NRS, and prealbumin, miR-22-3p offers unique advantages and some limitations. Unlike BMI, which can be confounded by factors such as edema or tumor mass, miR-22-3p is a molecular marker less likely to be influenced by these factors, providing a more direct measure of nutritional risk60. While SGA is a comprehensive but subjective tool, miR-22-3p offers an objective and reproducible measure that could complement SGA in assessing nutritional risk60. Though NRS-2002 is effective in identifying malnutrition risk, it may not predict the severity or progression of malnutrition as accurately as miR-22-3p, which also offers insights into the underlying biological processes61. Prealbumin, though commonly used, is affected by inflammation and liver function, whereas miR-22-3p, with its correlation to inflammatory markers like CRP, might offer a more stable alternative in chronic disease and cancer contexts26.
miR-22-3p offers specificity to molecular pathways involved in metabolism and inflammation, providing a direct link to processes leading to malnutrition43,44,45,46,47,48,49. Its potential for early detection allows for the identification of patients at risk of malnutrition before clinical signs appear, enabling earlier intervention. As a molecular marker, miR-22-3p provides an objective measure, unlike traditional tools that often rely on subjective assessments. However, the use of miR-22-3p is limited by the need for specialized laboratory equipment and expertise, which may not be available in all clinical settings, potentially restricting its widespread application. The lack of standardized cut-off values further complicates its clinical use, necessitating additional research to establish reliable thresholds. Additionally, the complexity of interpreting miR-22-3p levels, which requires a deeper understanding of molecular biology, could pose challenges for routine clinical implementation.
Our study is not exempt from limitations. These include its retrospective nature, a relatively small sample size, variations in treatment protocols (not all patients underwent surgery), the use of subjective tools such as SGA in assessing the nutritional status of patients, and inclusion of elderly individuals (up to 87 years old) who may have been affected by sarcopenia, potentially altering the clinical manifestation of cachexia. Furthermore, there was a lack of data on muscle mass assessed through densitometry, the analysis focused on a single miRNA (miRNA profiles might offer enhanced diagnostic utility), and the impact of the studied miRNA on their predicted target genes was not explored. Conversely, our study achieved homogeneity by exclusively enrolling patients with HNSCC undergoing a specific type of RT (IMRT), and ensured that all participants received a complete course of treatment.
Considering that our findings do not provide a comprehensive understanding of the role of miR-22-3p in the occurrence of nutritional deficiencies in HNC patients, and given the limited availability of literature on this miRNA, further investigations are warranted. Validating the predictive capacity of miR-22-3p requires assessment in a sufficiently large, prospective study cohort. Nonetheless, to our knowledge, this study represents the first evidence suggesting that miR-22-3p could serve as a biomarker for evaluating the risk of nutritional deficiencies in HNC patients undergoing IMRT. Pre-treatment expression levels of miR-22-3p may offer valuable insights into assessing the likelihood of poor nutritional status in HNC patients undergoing IMRT. Its integration into clinical practice could enhance the accuracy of nutritional assessments, particularly when used alongside traditional parameters. However, further research and standardization are needed to fully realize its potential in clinical settings. Investigating new biomarkers for nutritional deficiencies shows potential to improve diagnostic accuracy, expedite the application of nutritional and supportive treatments, and stimulate the advancement of innovative therapies.
Methods
Study group characteristics
The study group comprised 56 individuals with clinically confirmed advanced HNC (stages III-IV, specifically T4 or N0-3, or unresectable nodal disease, or deemed unfit for surgery), who received treatment at the Department of Oncology, Medical University of Lublin (2014 to 2017). The cancer stage was determined based on the 7th edition of the TNM classification. Inclusion criteria included age over 18 years old, any gender, and have a histopathological confirmed diagnosis of HNSCC. They underwent treatment using the IMRT technique either post-surgery or as their primary treatment, along with or without sequential and/or concomitant CTH, receiving the full prescribed radiation dose. Exclusion criteria encompassed tumor stage I-II, active infections, autoimmune disorders, prior history of any cancer, concurrent malignancies, and any form of anti-cancer therapy besides surgery. Written informed consent was obtained from all participants prior to their inclusion in the study. The Bioethical Commission of the Medical University of Lublin approved the study protocol (KE-0254/232/2014). All methods were carried out following the ethical guidelines of the 1975 Declaration of Helsinki (latest revision, 2013).
Treatment and patient assessment
The patients underwent treatment using the ONCOR (Siemens) IMRT technique. A dose range of 54–70 Gy was administered in 2 Gy daily fractions, 5 days per week, excluding Saturdays and Sundays. The sequential (SEQ) technique was used, with the initial lower-dose phase (weeks 1–5) followed by the high-dose boost volume phase (weeks 6–7), utilizing 2–3 separate dose plans. For patients in the advanced stages of cancer, 35 irradiation fractions were targeted at the tumor and enlarged lymph nodes (LNs), resulting in a total dose of 70 Gy. Postoperative patients with a higher risk received 33 fractions of irradiation, totaling 66 Gy, while those at average and low risk received doses of 60 Gy and 54 Gy, respectively. Elective LNs were treated with doses of 54–60 Gy. For postoperative patients, a total dose of 54–60 Gy was applied at 2 Gy per fraction. For definitive radiotherapy, the total dose of 66–70 Gy at 2 Gy per fraction was administered. Concurrent CRT included scheme: cisplatin 100 mg/m2 in 21-day cycles. Patients’ performance status was evaluated using the Eastern Cooperative Oncology Group (ECOG) scale. Alcohol consumption was assessed using the International Statistical Classification of Diseases (ICD) and Related Health Problems scales. In this study, nutritional deficiencies were defined as a broad spectrum of impairments with various endpoints, assessed using multiple tools, including NRS-2002, the WHO’s BMI-based definition of malnutrition, SGA, and CWL. Nutritional risk was evaluated using the NRS-200261. Diagnostic confirmation of malnutrition was based on a BMI < 18.5 kg/m² according to WHO criteria60. According to the SGA scale, the study group was divided into three categories: well-nourished patients (SGA-A), moderately malnourished patients (SGA-B), and severely malnourished patients (SGA-C)62. CWL was defined as a loss of > 5% from the beginning of RT to the 4th week or > 6.25% to the 7th week of therapy63. The nutritional status of the patients was assessed using the NRS and SGA scales prior to hospitalization, during physical examinations conducted consistently by the same medical doctor, in order to minimize assessment bias. Additionally, the group of smokers was further categorized into two subgroups: current and former smokers. A non-smoker was defined as an individual who has never smoked or who has smoked fewer than 100 cigarettes in their lifetime. Meanwhile, a current smoker or ex-smoker was identified as someone who has smoked more than 100 cigarettes in their life or is currently smoking.
miRNA expression analysis
Blood samples from all HNC patients for molecular and laboratory analysis (including total protein (TP), Albumin, Pre-albumin, Transferrin, and CRP) were collected prior to the commencement of RT. Additionally, miRNA relative expression levels and routine laboratory measurements were taken at the same timepoint, between 24 and 72 h before the start of RT. For miRNA analysis, 5 mL of peripheral blood was drawn from each patient using EDTA-containing tubes. The collected samples underwent centrifugation at 1000× g for 15 min. Within the subsequent 30 min, plasma was carefully collected and immediately processed for miRNA isolation. The miRNA isolation was conducted using a column-based method with a dedicated kit (miRNeasy Serum/Plasma Kit, Qiagen, Hilden, Germany) following the manufacturer’s protocol, utilizing 200 µL of plasma samples. The purified miRNA samples were then reverse transcribed into complementary DNA (cDNA) using a specialized kit (TaqMan Advanced miRNA cDNA Synthesis Kit, Thermo Fisher Scientific, Waltham, MA, USA). The amplification process was carried out on a StepOnePlus device (Applied Biosystems, Foster City, CA, USA) following the manufacturer’s instructions. For the real-time PCR, 96-well plates were employed, and each well had a total reaction volume of 20 µL. This included 10 µL of TaqMan Fast Advanced Master Mix (Thermo Fisher Scientific, USA), 1 µL of TaqMan Advanced miRNA Assay (20×) specifying hsa-miR-22-3p (Assay ID: 477985, Thermo Fisher Scientific, USA), 4 µL of RNase-free water, and 5 µL of diluted (1:10) cDNA template. The thermal cycling conditions involved enzyme activation at 95 °C, followed by 40 cycles of denaturation at 95 °C for 1 s, and annealing and elongation at 60 °C for 20 s. Each sample was analyzed in triplicate. The level of miRNA-22-3p expression was normalized to miR-26a-3p (Assay name: hsa-miR-26a-3p, Assay ID: 47995), Thermo Fisher Scientific, USA) as a reference assay using 2−∆∆Ct and 2−∆Ct formulae.
Bioelectrical impedance analysis
The body composition parameters were acquired through Bioelectrical Impedance Analysis (BIA), performed using the ImpediMed SFB7 BioImp device for bioimpedance analysis in Pinkenba, QLD, Australia.
Fat mass (FM) and fat-free mass (FFM) were calculated with the use of BIA, the FFMI and the normalized fat-free mass index (nFFMI) using the following formulae: FFMI [kg/m2] = FFM [kg]/(height [m])2 ; nFFMI [kg/m2] = FFMI [kg/m2] + 6.1 × (1.8 − height [m])64.
Statistical analysis
The statistical analysis of the data was conducted using MedCalc 15.8 software (MedCalc Software, Belgium). Results were deemed statistically significant at p < 0.05. As the continuous variables exhibited a non-normal distribution, non-parametric tests were employed whenever applicable. Spearman’s rank correlation was utilized to assess correlations between the levels of the studied miRNA and clinical, demographic, and nutritional variables. The Mann–Whitney U test was employed to compare the expression of the studied miRNA based on demographic, clinical variables, and nutritional status. ROC curve analysis was performed to establish cut-off points and evaluate the diagnostic utility of the studied miRNA in distinguishing patients with varying nutritional statuses. For each nutritional deficiency, “high” and “low” miR-22-3p expressions are defined based on the ROC-derived cut-off specific to that deficiency (presented in Table 2). In the case of survival a cut-off value for malnutrition according to SGA was used. Patients with miR-22-3p expression ≤5.28 were classified as having ‘low’ expression, while those with values >5.28 were classified as having ‘high’ expression. For the univariable assessment of the risk of underweight (BMI), malnutrition (SGA), nutritional risk (NRS-2002), and CWL concerning clinical, demographic, and epigenetic variables, odds ratios (OR) with a 95% confidence interval (95% CI) were calculated. Regarding multivariable analysis, logistic regression was utilized. The backward elimination method was employed to select statistically significant results from the univariable analysis for inclusion. Univariable OS analysis was conducted using the two-sided log-rank test, calculating hazard ratios (HR) and 95% CI, and visualizing the outcomes with Kaplan–Meier curves. For multivariable OS analysis, Cox proportional hazard models were used, again employing the backward elimination method to select statistically significant results from the univariable analysis for inclusion in the model.
Data availability
The data presented in this study are available on request from the corresponding author (KC).
References
-
Mody, M. D., Rocco, J. W., Yom, S. S., Haddad, R. I. & Saba, N. F. Head and neck cancer. Lancet 398, 2289–2299 (2021).
Google Scholar
-
Johnson, D. E. et al. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers 6, 92 (2020).
Google Scholar
-
Sturgis, E. M. & Cinciripini, P. M. Trends in head and neck cancer incidence in relation to smoking prevalence: An emerging epidemic of human papillomavirus-associated cancers? Cancer. 110, 1429–1435 (2007).
Google Scholar
-
Cohen, N., Fedewa, S. & Chen, A. Y. Epidemiology and demographics of the head and neck cancer population. Oral Maxillofac. Surg. Clin. North. Am. 30, 381–395 (2018).
Google Scholar
-
Eguchi, K. et al. Association between laryngopharyngeal reflux and radiation-induced mucositis in head and neck cancer. Anticancer Res. 38, 477–480 (2018).
Google Scholar
-
Sung, H. et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021).
Google Scholar
-
Abogunrin, S., Di Tanna, G. L., Keeping, S., Carroll, S. & Iheanacho, I. Prevalence of human papillomavirus in head and neck cancers in European populations: a meta-analysis. BMC Cancer. 14, 968 (2014).
Google Scholar
-
Chien, Y. C. et al. Serologic markers of Epstein-Barr virus infection and nasopharyngeal carcinoma in Taiwanese men. N Engl. J. Med. 345, 1877–1882 (2001).
Google Scholar
-
Pfister, P. D. G. et al. Head and neck cancers, Version 2.2020, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Canc Netw. 18, 873–898 (2020).
Google Scholar
-
Bonner, J. A. et al. Radiotherapy plus Cetuximab for squamous-cell carcinoma of the head and neck. N Engl. J. Med. 354, 567–578 (2006).
Google Scholar
-
Lee, N. Y. et al. Avelumab plus standard-of-care chemoradiotherapy versus chemoradiotherapy alone in patients with locally advanced squamous cell carcinoma of the head and neck: A randomised, double-blind, placebo-controlled, multicentre, phase 3 trial. Lancet Oncol. 22, 450–462 (2021).
Google Scholar
-
García-Peris, P. et al. Long-term prevalence of oropharyngeal dysphagia in head and neck cancer patients: Impact on quality of life. Clin. Nutr. 26, 710–717 (2007).
Google Scholar
-
Alshadwi, A. et al. Nutritional considerations for head and neck cancer patients: A review of the literature. J. Oral Maxillofac. Surg. 71, 1853–1860 (2013).
Google Scholar
-
Chasen, M. R. & Bhargava, R. A descriptive review of the factors contributing to nutritional compromise in patients with head and neck cancer. Support Care Cancer. 17, 1345–1351 (2009).
Google Scholar
-
Bischoff, S. C. et al. Standard operating procedures for ESPEN guidelines and consensus papers. Clin. Nutr. 34, 1043–1051 (2015).
Google Scholar
-
Castillo-Martínez, L. et al. Nutritional assessment tools for the identification of malnutrition and nutritional risk associated with cancer treatment. Rev. Invest. Clin. 70, 121–125 (2018).
Google Scholar
-
Cederholm, T. et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin. Nutr. 36, 49–64 (2017).
Google Scholar
-
Cederholm, T. et al. GLIM criteria for the diagnosis of malnutrition: A consensus report from the global clinical nutrition community. Clin. Nutr. 38, 1–9 (2019).
Google Scholar
-
Argilés, J. M., Busquets, S., Stemmler, B. & López-Soriano, F.J. Cancer cachexia: Understanding the molecular basis. Nat. Rev. Cancer. 14, 754–762 (2014).
Google Scholar
-
Stitt, T. N. et al. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol. Cell. 14, 395–403 (2004).
Google Scholar
-
Yoeli-Lerner, M. & Toker, A. Akt/PKB signaling in cancer: A function in cell motility and invasion. Cell Cycle 5, 603–605 (2006).
Google Scholar
-
Paul, P. K. & Kumar, A. TRAF6 coordinates the activation of autophagy and ubiquitin-proteasome systems in atrophying skeletal muscle. Autophagy. 7, 555–556 (2011).
Google Scholar
-
Christofides, A., Konstantinidou, E., Jani, C. & Boussiotis, V. A. The role of peroxisome proliferator-activated receptors (PPAR) in immune responses. Metabolism. 114, 154338 (2021).
Google Scholar
-
Stephens, N. A., Skipworth, R. J. & Fearon, K. C. Cachexia, survival and the acute phase response. Curr. Opin. Support Palliat. Care 2, 267–274 (2008).
Google Scholar
-
Johns, N. et al. Genetic basis of interindividual susceptibility to cancer cachexia: Selection of potential candidate gene polymorphisms for association studies. J. Genet. 93, 893–916 (2014).
Google Scholar
-
Chiang, H. C. et al. Transferrin and prealbumin identify esophageal Cancer patients with malnutrition and poor prognosis in patients with normal albuminemia: A cohort study. Nutr. Cancer 74, 3546–3555 (2022).
Google Scholar
-
Fatyga, P. et al. The relationship between malnutrition risk and inflammatory biomarkers in outpatient geriatric population. Eur. Geriatr. Med. 11, 383–391 (2020).
Google Scholar
-
28 Kaya, T. et al. Association between neutrophil-to-lymphocyte ratio and nutritional status in geriatric patients. J. Clin. Lab. Anal. 33, e22636 (2019).
Google Scholar
-
Wang, X. et al. Neutrophil-to-lymphocyte ratio is associated with malnutrition risk estimated by the Royal Free Hospital-Nutritional Prioritizing Tool in hospitalized cirrhosis. JPEN J. Parenter. Enter. Nutr. 46, 123–129 (2022).
Google Scholar
-
Li, Z. C. et al. Decreased expression of microRNA-130a correlates with TNF-α in the development of osteoarthritis. Int. J. Clin. Exp. Pathol. 8, 2555–2564 (2015).
Google Scholar
-
Zhang, J. et al. NF-κB-modulated miR-130a targets TNF-α in cervical cancer cells. J. Transl Med. 12, 155 (2014).
Google Scholar
-
Kulyté, A. et al. MicroRNA profiling links miR-378 to enhanced adipocyte lipolysis in human cancer cachexia. Am. J. Physiol. Endocrinol. Metab. 306, E267–E274 (2014).
Google Scholar
-
He, W. A. et al. Microvesicles containing miRNAs promote muscle cell death in cancer cachexia via TLR7. Proc. Natl. Acad. Sci. U. S. A. 111, 4525–4529 (2014).
-
Mazurek, M. et al. High mir-511-3p expression as a potential predictor of a poor nutritional status in head and neck cancer patients subjected to intensity-modulated radiation therapy. J. Clin. Med. 11, 805 (2022).
Google Scholar
-
Del Martínez-Jiménez, C., Méndez-Mancilla, V., Portales-Pérez, D. P. & A. & miRNAs in nutrition, obesity, and cancer: The biology of miRNAs in metabolic disorders and its relationship with cancer development. Mol. Nutr. Food Res. 62, 1600994 (2018).
Google Scholar
-
Powrózek, T. et al. miRNA-130a significantly improves accuracy of SGA nutritional assessment tool in prediction of malnutrition and cachexia in radiotherapy-treated head and neck cancer patients. Cancers (Basel) 10, 294 (2018).
Google Scholar
-
GeneCards. MIR22 Gene – microRNA 22. https://www.genecards.org/cgi-bin/carddisp.pl?gene=MIR22 (2024).
-
Gene, N. C. B. I. MIR22 microRNA 22 [Homo sapiens (human)]. https://www.ncbi.nlm.nih.gov/gene/407004.
-
Wang, Y., Lin, C. & Exosomes miR-22-3p derived from mesenchymal stem cells suppress colorectal cancer cell proliferation and invasion by regulating RAP2B and PI3K/AKT pathway. J. Oncol. 2021, 3874478 (2021).
-
Guan, B., Ma, J., Yang, Z., Yu, F. & Yao, J. LncRNA NCK1-AS1 exerts oncogenic property in gastric cancer by targeting the miR-22-3p/BCL9 axis to activate the Wnt/β-catenin signaling. Environ. Toxicol. (2021).
-
Wang, M., Chen, B., Ru, Z. & Cong, L. CircRNA circ-ITCH suppresses papillary thyroid cancer progression through miR-22-3p/CBL/β-catenin pathway. Biochem. Biophys. Res. Commun. 504, 283–288 (2018).
Google Scholar
-
Guo, Y., Zheng, H., Yin, J. & Wang, H. Rs4911154 of circ-ITCH aggravated tumor malignancy of thyroid nodules via the circ-ITCH/miR-22-3p/CBL axis. Sci. Rep. 11, 18491 (2021).
Google Scholar
-
Deng, X. et al. High expression of mir-22-3p in chicken hierarchical follicles promotes granulosa cell proliferation, steroidogenesis, and lipid metabolism via PTEN/PI3K/Akt/mTOR signaling pathway. Int. J. Biol. Macromol. 253 (Pt 7), 127415 (2023).
Google Scholar
-
Wang, Y. et al. MiR-22-3p and miR-29a-3p synergistically inhibit hepatic stellate cell activation by targeting AKT3. Exp. Biol. Med. (Maywood). 247, 1712–1731 (2022).
Google Scholar
-
Wang, J. et al. Long non-coding RNA FABP5P3/miR-22 axis improves TGFβ1-induced fatty acid oxidation deregulation and fibrotic changes in proximal tubular epithelial cells of renal fibrosis. Cell. Cycle. 22, 433–449 (2023).
Google Scholar
-
Yang, X. et al. MiR-22-3p suppresses cell growth via MET/STAT3 signaling in lung cancer. Am. J. Transl Res. 13, 1221–1232 (2021).
Google Scholar
-
Wen, W. et al. Mir-22-3p regulates muscle fiber-type conversion through inhibiting AMPK/SIRT1/PGC-1α pathway. Anim. Biotechnol. 32, 254–261 (2021).
Google Scholar
-
Wang, S. et al. MiR-22-3p inhibits proliferation and promotes differentiation of skeletal muscle cells by targeting IGFBP3 in Hu Sheep. Anim. (Basel). 12, 114 (2022).
Google Scholar
-
Li, R. et al. Long non-coding RNA Mir22hg-derived mir-22-3p promotes skeletal muscle differentiation and regeneration by inhibiting HDAC4. Mol. Ther. Nucleic Acids. 24, 200–211 (2021).
Google Scholar
-
Jiang, R., Lönnerdal, B. & Milk-Derived Mir-22-3p promotes proliferation of human intestinal epithelial cells (HIECs) by regulating Gene expression. Nutrients. 14, 4901 (2022).
Google Scholar
-
Lin, Y. et al. mir-22-3p/KLF6/MMP14 axis in fibro-adipogenic progenitors regulates fatty infiltration in muscle degeneration. FASEB J. 34, 12691–12701 (2020).
Google Scholar
-
Senese, R. et al. Mir-22-3p is involved in gluconeogenic pathway modulated by 3,5-diiodo-L-thyronine (T2). Sci. Rep. 9, 16645 (2019).
Google Scholar
-
Lee, D. E. et al. Cancer cachexia-induced muscle atrophy: Evidence for alterations in microRNAs important for muscle size. Physiol. Genomics. 49, 253–260 (2017).
Google Scholar
-
Takahashi, K., Jia, H. & Takahashi, S. Comprehensive miRNA and DNA microarray analyses reveal the response of hepatic miR-203 and its target gene to protein malnutrition in rats. Genes (Basel). 13, 75 (2021).
Google Scholar
-
Qiu, L. et al. Exosomes of oral squamous cell carcinoma cells containing miR-181a-3p induce muscle cell atrophy and apoptosis by transmissible endoplasmic reticulum stress signaling. Biochem. Biophys. Res. Commun. 533, 831–837 (2020).
Google Scholar
-
Chern, C. L., Huang, R. F., Chen, Y. H., Cheng, J. T. & Liu, T. Z. Folate deficiency-induced oxidative stress and apoptosis are mediated via homocysteine-dependent overproduction of hydrogen peroxide and enhanced activation of NF-kappaB in human hep G2 cells. Biomed. Pharmacother. 55, 434–442 (2001).
Google Scholar
-
Li, C. et al. MicroRNA-22-3p and MicroRNA-149-5p inhibit human hepatocellular carcinoma cell growth and metastasis properties by regulating methylenetetrahydrofolate reductase. Curr. Issues Mol. Biol. 44, 952–962 (2022).
Google Scholar
-
Okugawa, Y. et al. Prognostic impact of Sarcopenia and its correlation with circulating miR-21 in colorectal cancer patients. Oncol. Rep. 39, 1555–1564 (2018).
Google Scholar
-
Ferreira, T. J. et al. Dietary intake is associated with miR-31 and miR-375 expression in patients with head and neck squamous cell carcinoma. Nutr. Cancer. 74, 2049–2058 (2022).
Google Scholar
-
Trabulo, C. et al. Assessment of nutritional status of oncology patients at hospital admission: A Portuguese real-world study. Front. Nutr. 9, 972525 (2022).
Google Scholar
-
Orell-Kotikangas, H. et al. NRS-2002 for pre-treatment nutritional risk screening and nutritional status assessment in head and neck cancer patients. Support Care Cancer. 23, 1495–1502 (2015).
Google Scholar
-
Susetyowati, S., Kurniasari, F. N., Sholikhati, A. S., Hardianti, M. & Ekaputra, E. Assessment of nutritional status in patients with head and neck cancer before radiotherapy: A single-center, cross-sectional study. Medeni Med. J. 39, 24–32 (2024).
Google Scholar
-
Zeng, Q. et al. Critical weight loss predicts poor prognosis in nasopharyngeal carcinoma. BMC Cancer 16, 169 (2016).
Google Scholar
-
Kohli, K. et al. A bioimpedance analysis of head-and-neck cancer patients undergoing radiotherapy. Curr. Oncol. 25, e193–e199 (2018).
Google Scholar
Author information
Authors and Affiliations
Contributions
KC, IHM, AB, TMM and RM conceived the concept of the study. IHM, MM, AB, TMM and RM contributed to the research design. MM performed miRNA expression analysis. EP, MM and AB were involved in data collection. IHM, MM, RM analyzed the data. KC, IHM, MM, AB and RM wrote the manuscript. All authors edited and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
Reprints and permissions
About this article
Cite this article
Chawrylak, K., Homa-Mlak, I., Mazurek, M. et al. MiR-22-3p as a promising predictor of nutritional deficiencies in patients with head and neck cancer subjected to intensity-modulated radiation therapy.
Sci Rep 14, 28120 (2024). https://doi.org/10.1038/s41598-024-79641-3
-
Received: 21 June 2024
-
Accepted: 11 November 2024
-
Published: 15 November 2024
-
DOI: https://doi.org/10.1038/s41598-024-79641-3
Keywords
- Head and neck cancer
- Malnutrition
- Intensity-modulated radiation therapy
- miR-22-3p
- Nutritional deficiencies
- Critical weight loss