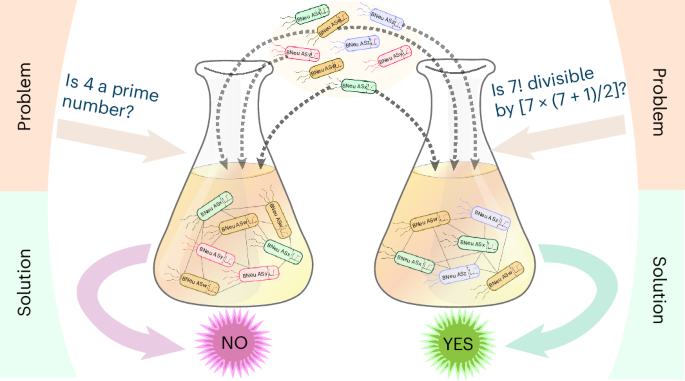
Abstract
Here, we report a modular multicellular system created by mixing and matching discrete engineered bacterial cells. This system can be designed to solve multiple computational decision problems. The modular system is based on a set of engineered bacteria that are modeled as an ‘artificial neurosynapse’ that, in a coculture, formed a single-layer artificial neural network-type architecture that can perform computational tasks. As a demonstration, we constructed devices that function as a full subtractor and a full adder. The system is also capable of solving problems such as determining if a number between 0 and 9 is a prime number and if a letter between A and L is a vowel. Finally, we built a system that determines the maximum number of pieces of a pie that can be made for a given number of straight cuts. This work may have importance in biocomputer technology development and multicellular synthetic biology.

This is a preview of subscription content, access via your institution
Access options
style{display:none!important}.LiveAreaSection-193358632 *{align-content:stretch;align-items:stretch;align-self:auto;animation-delay:0s;animation-direction:normal;animation-duration:0s;animation-fill-mode:none;animation-iteration-count:1;animation-name:none;animation-play-state:running;animation-timing-function:ease;azimuth:center;backface-visibility:visible;background-attachment:scroll;background-blend-mode:normal;background-clip:borderBox;background-color:transparent;background-image:none;background-origin:paddingBox;background-position:0 0;background-repeat:repeat;background-size:auto auto;block-size:auto;border-block-end-color:currentcolor;border-block-end-style:none;border-block-end-width:medium;border-block-start-color:currentcolor;border-block-start-style:none;border-block-start-width:medium;border-bottom-color:currentcolor;border-bottom-left-radius:0;border-bottom-right-radius:0;border-bottom-style:none;border-bottom-width:medium;border-collapse:separate;border-image-outset:0s;border-image-repeat:stretch;border-image-slice:100%;border-image-source:none;border-image-width:1;border-inline-end-color:currentcolor;border-inline-end-style:none;border-inline-end-width:medium;border-inline-start-color:currentcolor;border-inline-start-style:none;border-inline-start-width:medium;border-left-color:currentcolor;border-left-style:none;border-left-width:medium;border-right-color:currentcolor;border-right-style:none;border-right-width:medium;border-spacing:0;border-top-color:currentcolor;border-top-left-radius:0;border-top-right-radius:0;border-top-style:none;border-top-width:medium;bottom:auto;box-decoration-break:slice;box-shadow:none;box-sizing:border-box;break-after:auto;break-before:auto;break-inside:auto;caption-side:top;caret-color:auto;clear:none;clip:auto;clip-path:none;color:initial;column-count:auto;column-fill:balance;column-gap:normal;column-rule-color:currentcolor;column-rule-style:none;column-rule-width:medium;column-span:none;column-width:auto;content:normal;counter-increment:none;counter-reset:none;cursor:auto;display:inline;empty-cells:show;filter:none;flex-basis:auto;flex-direction:row;flex-grow:0;flex-shrink:1;flex-wrap:nowrap;float:none;font-family:initial;font-feature-settings:normal;font-kerning:auto;font-language-override:normal;font-size:medium;font-size-adjust:none;font-stretch:normal;font-style:normal;font-synthesis:weight style;font-variant:normal;font-variant-alternates:normal;font-variant-caps:normal;font-variant-east-asian:normal;font-variant-ligatures:normal;font-variant-numeric:normal;font-variant-position:normal;font-weight:400;grid-auto-columns:auto;grid-auto-flow:row;grid-auto-rows:auto;grid-column-end:auto;grid-column-gap:0;grid-column-start:auto;grid-row-end:auto;grid-row-gap:0;grid-row-start:auto;grid-template-areas:none;grid-template-columns:none;grid-template-rows:none;height:auto;hyphens:manual;image-orientation:0deg;image-rendering:auto;image-resolution:1dppx;ime-mode:auto;inline-size:auto;isolation:auto;justify-content:flexStart;left:auto;letter-spacing:normal;line-break:auto;line-height:normal;list-style-image:none;list-style-position:outside;list-style-type:disc;margin-block-end:0;margin-block-start:0;margin-bottom:0;margin-inline-end:0;margin-inline-start:0;margin-left:0;margin-right:0;margin-top:0;mask-clip:borderBox;mask-composite:add;mask-image:none;mask-mode:matchSource;mask-origin:borderBox;mask-position:0 0;mask-repeat:repeat;mask-size:auto;mask-type:luminance;max-height:none;max-width:none;min-block-size:0;min-height:0;min-inline-size:0;min-width:0;mix-blend-mode:normal;object-fit:fill;object-position:50% 50%;offset-block-end:auto;offset-block-start:auto;offset-inline-end:auto;offset-inline-start:auto;opacity:1;order:0;orphans:2;outline-color:initial;outline-offset:0;outline-style:none;outline-width:medium;overflow:visible;overflow-wrap:normal;overflow-x:visible;overflow-y:visible;padding-block-end:0;padding-block-start:0;padding-bottom:0;padding-inline-end:0;padding-inline-start:0;padding-left:0;padding-right:0;padding-top:0;page-break-after:auto;page-break-before:auto;page-break-inside:auto;perspective:none;perspective-origin:50% 50%;pointer-events:auto;position:static;quotes:initial;resize:none;right:auto;ruby-align:spaceAround;ruby-merge:separate;ruby-position:over;scroll-behavior:auto;scroll-snap-coordinate:none;scroll-snap-destination:0 0;scroll-snap-points-x:none;scroll-snap-points-y:none;scroll-snap-type:none;shape-image-threshold:0;shape-margin:0;shape-outside:none;tab-size:8;table-layout:auto;text-align:initial;text-align-last:auto;text-combine-upright:none;text-decoration-color:currentcolor;text-decoration-line:none;text-decoration-style:solid;text-emphasis-color:currentcolor;text-emphasis-position:over right;text-emphasis-style:none;text-indent:0;text-justify:auto;text-orientation:mixed;text-overflow:clip;text-rendering:auto;text-shadow:none;text-transform:none;text-underline-position:auto;top:auto;touch-action:auto;transform:none;transform-box:borderBox;transform-origin:50% 50%0;transform-style:flat;transition-delay:0s;transition-duration:0s;transition-property:all;transition-timing-function:ease;vertical-align:baseline;visibility:visible;white-space:normal;widows:2;width:auto;will-change:auto;word-break:normal;word-spacing:normal;word-wrap:normal;writing-mode:horizontalTb;z-index:auto;-webkit-appearance:none;-moz-appearance:none;-ms-appearance:none;appearance:none;margin:0}.LiveAreaSection-193358632{width:100%}.LiveAreaSection-193358632 .login-option-buybox{display:block;width:100%;font-size:17px;line-height:30px;color:#222;padding-top:30px;font-family:Harding,Palatino,serif}.LiveAreaSection-193358632 .additional-access-options{display:block;font-weight:700;font-size:17px;line-height:30px;color:#222;font-family:Harding,Palatino,serif}.LiveAreaSection-193358632 .additional-login>li:not(:first-child)::before{transform:translateY(-50%);content:””;height:1rem;position:absolute;top:50%;left:0;border-left:2px solid #999}.LiveAreaSection-193358632 .additional-login>li:not(:first-child){padding-left:10px}.LiveAreaSection-193358632 .additional-login>li{display:inline-block;position:relative;vertical-align:middle;padding-right:10px}.BuyBoxSection-683559780{display:flex;flex-wrap:wrap;flex:1;flex-direction:row-reverse;margin:-30px -15px 0}.BuyBoxSection-683559780 .box-inner{width:100%;height:100%;padding:30px 5px;display:flex;flex-direction:column;justify-content:space-between}.BuyBoxSection-683559780 p{margin:0}.BuyBoxSection-683559780 .readcube-buybox{background-color:#f3f3f3;flex-shrink:1;flex-grow:1;flex-basis:255px;background-clip:content-box;padding:0 15px;margin-top:30px}.BuyBoxSection-683559780 .subscribe-buybox{background-color:#f3f3f3;flex-shrink:1;flex-grow:4;flex-basis:300px;background-clip:content-box;padding:0 15px;margin-top:30px}.BuyBoxSection-683559780 .subscribe-buybox-nature-plus{background-color:#f3f3f3;flex-shrink:1;flex-grow:4;flex-basis:100%;background-clip:content-box;padding:0 15px;margin-top:30px}.BuyBoxSection-683559780 .title-readcube,.BuyBoxSection-683559780 .title-buybox{display:block;margin:0;margin-right:10%;margin-left:10%;font-size:24px;line-height:32px;color:#222;text-align:center;font-family:Harding,Palatino,serif}.BuyBoxSection-683559780 .title-asia-buybox{display:block;margin:0;margin-right:5%;margin-left:5%;font-size:24px;line-height:32px;color:#222;text-align:center;font-family:Harding,Palatino,serif}.BuyBoxSection-683559780 .asia-link{color:#069;cursor:pointer;text-decoration:none;font-size:1.05em;font-family:-apple-system,BlinkMacSystemFont,”Segoe UI”,Roboto,Oxygen-Sans,Ubuntu,Cantarell,”Helvetica Neue”,sans-serif;line-height:1.05em6}.BuyBoxSection-683559780 .access-readcube{display:block;margin:0;margin-right:10%;margin-left:10%;font-size:14px;color:#222;padding-top:10px;text-align:center;font-family:-apple-system,BlinkMacSystemFont,”Segoe UI”,Roboto,Oxygen-Sans,Ubuntu,Cantarell,”Helvetica Neue”,sans-serif;line-height:20px}.BuyBoxSection-683559780 ul{margin:0}.BuyBoxSection-683559780 .link-usp{display:list-item;margin:0;margin-left:20px;padding-top:6px;list-style-position:inside}.BuyBoxSection-683559780 .link-usp span{font-size:14px;color:#222;font-family:-apple-system,BlinkMacSystemFont,”Segoe UI”,Roboto,Oxygen-Sans,Ubuntu,Cantarell,”Helvetica Neue”,sans-serif;line-height:20px}.BuyBoxSection-683559780 .access-asia-buybox{display:block;margin:0;margin-right:5%;margin-left:5%;font-size:14px;color:#222;padding-top:10px;text-align:center;font-family:-apple-system,BlinkMacSystemFont,”Segoe UI”,Roboto,Oxygen-Sans,Ubuntu,Cantarell,”Helvetica Neue”,sans-serif;line-height:20px}.BuyBoxSection-683559780 .access-buybox{display:block;margin:0;margin-right:10%;margin-left:10%;font-size:14px;color:#222;opacity:.8px;padding-top:10px;text-align:center;font-family:-apple-system,BlinkMacSystemFont,”Segoe UI”,Roboto,Oxygen-Sans,Ubuntu,Cantarell,”Helvetica Neue”,sans-serif;line-height:20px}.BuyBoxSection-683559780 .price-buybox{display:block;font-size:30px;color:#222;font-family:-apple-system,BlinkMacSystemFont,”Segoe UI”,Roboto,Oxygen-Sans,Ubuntu,Cantarell,”Helvetica Neue”,sans-serif;padding-top:30px;text-align:center}.BuyBoxSection-683559780 .price-buybox-to{display:block;font-size:30px;color:#222;font-family:-apple-system,BlinkMacSystemFont,”Segoe UI”,Roboto,Oxygen-Sans,Ubuntu,Cantarell,”Helvetica Neue”,sans-serif;text-align:center}.BuyBoxSection-683559780 .price-info-text{font-size:16px;padding-right:10px;color:#222;font-family:-apple-system,BlinkMacSystemFont,”Segoe UI”,Roboto,Oxygen-Sans,Ubuntu,Cantarell,”Helvetica Neue”,sans-serif}.BuyBoxSection-683559780 .price-value{font-size:30px;font-family:-apple-system,BlinkMacSystemFont,”Segoe UI”,Roboto,Oxygen-Sans,Ubuntu,Cantarell,”Helvetica Neue”,sans-serif}.BuyBoxSection-683559780 .price-per-period{font-family:-apple-system,BlinkMacSystemFont,”Segoe UI”,Roboto,Oxygen-Sans,Ubuntu,Cantarell,”Helvetica Neue”,sans-serif}.BuyBoxSection-683559780 .price-from{font-size:14px;padding-right:10px;color:#222;font-family:-apple-system,BlinkMacSystemFont,”Segoe UI”,Roboto,Oxygen-Sans,Ubuntu,Cantarell,”Helvetica Neue”,sans-serif;line-height:20px}.BuyBoxSection-683559780 .issue-buybox{display:block;font-size:13px;text-align:center;color:#222;font-family:-apple-system,BlinkMacSystemFont,”Segoe UI”,Roboto,Oxygen-Sans,Ubuntu,Cantarell,”Helvetica Neue”,sans-serif;line-height:19px}.BuyBoxSection-683559780 .no-price-buybox{display:block;font-size:13px;line-height:18px;text-align:center;padding-right:10%;padding-left:10%;padding-bottom:20px;padding-top:30px;color:#222;font-family:-apple-system,BlinkMacSystemFont,”Segoe UI”,Roboto,Oxygen-Sans,Ubuntu,Cantarell,”Helvetica Neue”,sans-serif}.BuyBoxSection-683559780 .vat-buybox{display:block;margin-top:5px;margin-right:20%;margin-left:20%;font-size:11px;color:#222;padding-top:10px;padding-bottom:15px;text-align:center;font-family:-apple-system,BlinkMacSystemFont,”Segoe UI”,Roboto,Oxygen-Sans,Ubuntu,Cantarell,”Helvetica Neue”,sans-serif;line-height:17px}.BuyBoxSection-683559780 .tax-buybox{display:block;width:100%;color:#222;padding:20px 16px;text-align:center;font-family:-apple-system,BlinkMacSystemFont,”Segoe UI”,Roboto,Oxygen-Sans,Ubuntu,Cantarell,”Helvetica Neue”,sans-serif;line-height:NaNpx}.BuyBoxSection-683559780 .button-container{display:flex;padding-right:20px;padding-left:20px;justify-content:center}.BuyBoxSection-683559780 .button-container>*{flex:1px}.BuyBoxSection-683559780 .button-container>a:hover,.Button-505204839:hover,.Button-1078489254:hover,.Button-2737859108:hover{text-decoration:none}.BuyBoxSection-683559780 .btn-secondary{background:#fff}.BuyBoxSection-683559780 .button-asia{background:#069;border:1px solid #069;border-radius:0;cursor:pointer;display:block;padding:9px;outline:0;text-align:center;text-decoration:none;min-width:80px;margin-top:75px}.BuyBoxSection-683559780 .button-label-asia,.ButtonLabel-3869432492,.ButtonLabel-3296148077,.ButtonLabel-1636778223{display:block;color:#fff;font-size:17px;line-height:20px;font-family:-apple-system,BlinkMacSystemFont,”Segoe UI”,Roboto,Oxygen-Sans,Ubuntu,Cantarell,”Helvetica Neue”,sans-serif;text-align:center;text-decoration:none;cursor:pointer}.Button-505204839,.Button-1078489254,.Button-2737859108{background:#069;border:1px solid #069;border-radius:0;cursor:pointer;display:block;padding:9px;outline:0;text-align:center;text-decoration:none;min-width:80px;max-width:320px;margin-top:20px}.Button-505204839 .btn-secondary-label,.Button-1078489254 .btn-secondary-label,.Button-2737859108 .btn-secondary-label{color:#069}
/* style specs end */
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Synthetic neuromorphic computing in living cells
Synthetic neural-like computing in microbial consortia for pattern recognition
Pathways to cellular supremacy in biocomputing
Data availability
All relevant data supporting the findings are available within the paper, Extended Data figures and the Supplementary Data. Source data are provided with this paper.
References
-
Benenson, Y. Biomolecular computing systems: principles, progress and potential. Nat. Rev. Genet. 13, 455–468 (2012).
Google Scholar
-
Reif, J. H., Hauser, M., Pirrung, M. & LaBean, T. in Complex Systems Science in Biomedicine (eds. Deisboeck, T. S. & Kresh, J. Y.) 701–735 (Springer, 2006).
-
Ma, Q. et al. DNA computing: principle, construction, and applications in intelligent diagnostic. Small Struct. 2, 2100051 (2021).
Google Scholar
-
Yoon, J., Lim, J., Shin, M., Lee, T. & Choi, J. W. Toward bioelectronic device based on bionanohybrid composed of nanomaterials and biomaterials: from nucleic acid and protein to living cell. Appl. Phys. Rev. 10, 011302 (2023).
Google Scholar
-
Fan, D., Wang, J., Wang, E. & Dong, S. Propelling DNA computing with materials’ power: recent advancements in innovative DNA logic computing systems and smart bio‐applications. Adv. Sci. 7, 2001766 (2020).
Google Scholar
-
Lin, K. N., Volkel, K., Tuck, J. M. & Keung, A. J. Dynamic and scalable DNA-based information storage. Nat. Commun. 11, 2981 (2020).
Google Scholar
-
Lederman, H., Macdonald, J., Stefanovic, D. & Stojanovic, M. N. Deoxyribozyme-based three-input logic gates and construction of a molecular full adder. Biochemistry 45, 1194–1199 (2006).
Google Scholar
-
Fratto, B. E., Lewer, J. M. & Katz, E. An enzyme based half-adder and half-subtractor with a modular design. ChemPhysChem 17, 2210–2217 (2016).
Google Scholar
-
Cherry, K. M. & Qian, L. Scaling up molecular pattern recognition with DNA-based winner-take-all neural networks. Nature 559, 370–376 (2018).
Google Scholar
-
Adleman, L. M. Molecular computation of solutions to combinatorial problems. Science 266, 1021–1024 (1994).
Google Scholar
-
Faulhammer, D., Cukras, A. R., Lipton, R. J. & Landweber, L. F. Molecular computation: RNA solutions to chess problems. Proc. Natl Acad. Sci. USA 97, 1385–1389 (2000).
Google Scholar
-
Chao, J. et al. Solving mazes with single-molecule DNA navigators. Nat. Mater. 18, 273–279 (2018).
Google Scholar
-
Brophy, J. A. N. & Voigt, C. A. Principles of genetic circuit design. Nat. Methods 11, 508–520 (2014).
Google Scholar
-
Bonnet, J., Yin, P., Ortiz, M. E., Subsoontorn, P. & Endy, D. Amplifying genetic logic gates. Science 340, 599–603 (2013).
Google Scholar
-
Bonnerjee, D., Mukhopadhyay, S. & Bagh, S. Design, fabrication, and device chemistry of a 3-input-3-output synthetic genetic combinatorial logic circuit with a 3-input AND gate in a single bacterial cell. Bioconjug. Chem. 30, 3013–3020 (2019).
Google Scholar
-
Can, U. I., Nagarajan, N., Vural, D. C. & Zorlutuna, P. Muscle-cell-based ‘living diodes’. Adv. Biosyst. 1, 1600035 (2017).
Google Scholar
-
Wong, A., Wang, H., Poh, C. L. & Kitney, R. I. Layering genetic circuits to build a single cell, bacterial half adder. BMC Biol. 13, 40 (2015).
Google Scholar
-
Friedland, A. E. et al. Synthetic gene networks that count. Science 324, 1199–1202 (2009).
Google Scholar
-
Sexton, J. T. & Tabor, J. J. Multiplexing cell–cell communication. Mol. Syst. Biol. 16, e9618 (2020).
Google Scholar
-
Ausländer, D. et al. Programmable full-adder computations in communicating three-dimensional cell cultures. Nat. Methods 15, 57–60 (2018).
Google Scholar
-
Weinberg, B. et al. Large-scale design of robust genetic circuits with multiple inputs and outputs for mammalian cells. Nat. Biotechnol. 35, 453–462 (2017).
Google Scholar
-
Müller, M. et al. Designed cell consortia as fragrance-programmable analog-to-digital converters. Nat. Chem. Biol. 13, 309–316 (2017).
Google Scholar
-
Kim, M., Julius, A. A. & Cheang U. K. Microbiorobotics: Biologically Inspired Microscale Robotic Systems 2nd edn (Elsevier, 2017)
-
Justus, K. B. et al. A biosensing soft robot: autonomous parsing of chemical signals through integrated organic and inorganic interfaces. Sci. Robot. 4, eaax0765 (2019).
Google Scholar
-
Grozinger, L. et al. Pathways to cellular supremacy in biocomputing. Nat. Commun. 10, 5250 (2019).
Google Scholar
-
Chen, Y. Y. & Smolke, C. D. From DNA to targeted therapeutics: bringing synthetic biology to the clinic. Sci. Transl. Med. 3, 106ps42 (2011).
Google Scholar
-
Yan, X., Liu, X., Zhao, C. & Chen, G. Q. Applications of synthetic biology in medical and pharmaceutical fields. Signal Transduct. Target. Ther. 8, 199 (2023).
Google Scholar
-
Johnson, M. B., March, A. R. & Morsut, L. Engineering multicellular systems: using synthetic biology to control tissue self-organization. Curr. Opin. Biomed. Eng. 4, 163–173 (2017).
Google Scholar
-
Teravest, M. A., Li, Z. & Angenent, L. T. Bacteria-based biocomputing with cellular computing circuits to sense, decide, signal, and act. Energy Environ. Sci. 4, 4907–4916 (2011).
Google Scholar
-
Dvořák, P., Nikel, P. I., Damborský, J. & de Lorenzo, V. Bioremediation 3.0: engineering pollutant-removing bacteria in the times of systemic biology. Biotechnol. Adv. 35, 845–866 (2017).
Google Scholar
-
Gilbert, C. & Ellis, T. Biological engineered living materials: growing functional materials with genetically programmable properties. ACS Synth. Biol. 8, 1–15 (2019).
Google Scholar
-
Sarkar, K., Chakraborty, S., Bonnerjee, D. & Bagh, S. Distributed computing with engineered bacteria and its application in solving chemically generated 2 × 2 maze problems. ACS Synth. Biol. 10, 2456–2464 (2021).
Google Scholar
-
Collins, J. Synthetic biology: bits and pieces come to life. Nature 483, S8–S10 (2012).
Google Scholar
-
Ren, X. et al. Cardiac muscle cell-based coupled oscillator network for collective computing. Adv. Intell. Syst. 3, 2000253 (2021).
Google Scholar
-
Ji, J. et al. Large-scale cardiac muscle cell-based coupled oscillator network for vertex coloring problem. Adv. Intell. Syst. 5, 2200356 (2023).
Google Scholar
-
Sarkar, K., Bonnerjee, D., Srivastava, R. & Bagh, S. A single layer artificial neural network type architecture with molecular engineered bacteria for reversible and irreversible computing. Chem. Sci. 12, 15821–15832 (2021).
Google Scholar
-
Srivastava, R. & Bagh, S. A logically reversible double Feynman gate with molecular engineered bacteria arranged in an artificial neural network-type architecture. ACS Synth. Biol. 12, 51–60 (2023).
Google Scholar
-
Rizik, L., Danial, L., Habib, M., Weiss, R. & Daniel, R. Synthetic neuromorphic computing in living cells. Nat. Commun. 13, 5602 (2022).
Google Scholar
-
Millacura, F. A., Largey, B. & French, C. E. ParAlleL: a novel population-based approach to biological logic gates. Front. Bioeng. Biotechnol. 7, 46 (2019).
Google Scholar
-
Demuth, H. & De Jesús, B. Neural Network Design 2nd edn (Martin Hagan, 2014).
-
Sarkar, K., Mukhopadhyay, S., Bonnerjee, D., Srivastava, R. & Bagh, S. A frame-shifted gene, which rescued its function by non-natural start codons and its application in constructing synthetic gene circuits. J. Biol. Eng. 13, 20 (2019).
Google Scholar
-
Lutz, R. & Bujard, H. Independent and tight regulation of transcriptional units in Escherichia coli via the LacR/O, the TetR/O and AraC/I 1-I 2 regulatory elements. Nucleic Acids Res. 25, 1203–1210 (1997).
Google Scholar
-
Goldreich, O. Computational Complexity: A Conceptual Perspective (Cambridge Univ. Press, 2008).
-
Mezard, M. & Montanari, A. Information, Physics and Computation (Oxford Graduate Texts, Oxford Univ. Press, 2009).
-
Baldwin, C. Y. & Clark, K. B. in Complex Engineered Systems (eds. Braha, D. et al.) 175–205 (Springer, 2006).
-
Glykofrydis, F. & Elfick, A. Exploring standards for multicellular mammalian synthetic biology. Trends Biotechnol. 40, 1299–1312 (2022).
Google Scholar
-
Boo, A., Amaro, R. L. & Stan, G. B. Quorum sensing in synthetic biology: a review. Curr. Opin. Syst. Biol. 28, 100378 (2021).
Google Scholar
-
Ohlendorf, R., Vidavski, R. R., Eldar, A., Moffat, K. & Möglich, A. From dusk till dawn: one-plasmid systems for light-regulated gene expression. J. Mol. Biol. 416, 534–542 (2012).
Google Scholar
-
Multamäki, E. et al. Optogenetic control of bacterial expression by red light. ACS Synth. Biol. 11, 3354–3367 (2022).
Google Scholar
-
Macia, J., Vidiella, B. & Solé, R. V. Synthetic associative learning in engineered multicellular consortia. J. R. Soc. Interface 14, 20170158 (2017).
-
Moškon, M., Pušnik, Ž., Zimic, N. & Mraz, M. Field-programmable biological circuits and configurable (bio)logic blocks for distributed biological computing. Comput. Biol. Med. 128, 104109 (2021).
Google Scholar
-
Beal, J. et al. The long journey towards standards for engineering biosystems. EMBO Rep. 21, e50521 (2020).
Google Scholar
-
Toda, S., Blauch, L. R., Tang, S. K. Y., Morsut, L. & Lim, W. A. Programming self-organizing multicellular structures with synthetic cell–cell signaling. Science 361, 156–162 (2018).
Google Scholar
-
Aydin, O. et al. Principles for the design of multicellular engineered living systems. APL Bioeng. 6, 010903 (2022).
Google Scholar
-
Mukhopadhyay, S., Sarkar, K., Srivastava, R., Pal, A. & Bagh, S. Processing two environmental chemical signals with a synthetic genetic IMPLY gate, a 2-input-2-output integrated logic circuit, and a process pipeline to optimize its systems chemistry in Escherichia coli. Biotechnol. Bioeng. 117, 1502–1512 (2020).
Google Scholar
-
Salis, H. M., Mirsky, E. A. & Voigt, C. A. Automated design of synthetic ribosome binding sites to control protein expression. Nat. Biotechnol. 27, 946–950 (2009).
Google Scholar
Acknowledgements
This work was financially supported by grant RSI4002, Department of Atomic Energy, Government of India, awarded to Saha Institute of Nuclear Physics, and S.B. is a part of it.
Author information
Authors and Affiliations
Contributions
D.B., S.C., B.M., R.B. and A.P. performed all the experiments. D.B., S.C., B.M., R.B. and S.B. analyzed the data. D.B., S.C. and S.B. designed the experiments and wrote the manuscript. S.B. designed and conceived the study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemical Biology thanks Allen Liu, Pinar Zorlutuna and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Genetic circuit designs.
Generic maps of the synthetic genetic networks that encode corresponding specific functions to each bactoneuron type.
Extended Data Fig. 2 Details of characterization and Dose Responses experiments of Bactoneurons that went through no iteration and were selected as final.
The bactoneurons AS1, AS2, AS3, AS4, AS8 and AS9 were separately subjected to all possible combinations of input conditions (saturated concentration ‘1’ or absent ‘0’) yielding corresponding parameter values of Fold Change (F.C.), Σ Leakage (ΣL) and Signal variation (S.V.) (shown in figure). ‘Signal variation’ (S.V.) is a comparative parameter drawn by scaling the fluorescence signal (@ ‘ON’ state) of all bactoneurons in an iteration set (with the same output channel) by considering the strongest signal of all bactoneurons as ‘1’. For Dose response, the concentration of one parameter was varied across various concentration points while the other two inducers were kept constant at ‘0’ for repressor or saturated concentration for activator (specified atop each dose response curve). The fluorescence readings obtained from the dose response experiments were fitted with the Log-Sigmoid function (see Methods) to derive the parameters Bias (BAS#) and Weights (Winducer) and the respective Inducer Saturation points (Sat.) were identified. Each data point represent mean and s.d. from four independent colonies.
Source data
Extended Data Fig. 3 Details of characterization and Dose Responses experiments across iterations of Bactoneuron 5.
AS5A, AS5B, AS5C, AS5D, AS5E, AS5F and AS5G; yielding corresponding parameter values (shown in figure) of Fold Change (F.C.), Σ Leakage (ΣL), Signal variation (S.V.), Bias (BAS#), Weights (Winducer) and Inducer Saturation points (Sat.). The fluorescence readings obtained from the dose response experiments were fitted with the Log-Sigmoid function (see Methods) to derive the Weight and Bias parameters. The induction states of the two constant inducers are specified atop the fitted curves. The final selected Construct is shown boxed. Each data point represent mean and s.d. from four independent colonies.
Source data
Extended Data Fig. 4 Details of characterization and Dose Responses experiments across iterations of Bactoneuron 6.
AS6A, AS6B, AS6C, AS6D, AS6E and AS6F; yielding corresponding parameter values (shown in figure) of Fold Change (F.C.), Σ Leakage (ΣL), Signal variation (S.V.), Bias (BAS#), Weights (WInducer) and Inducer Saturation points (Sat.). The fluorescence readings obtained from the dose response experiments were fitted with the Log-Sigmoid function (see Methods) to derive the Weight and Bias parameters. The induction states of the two constant inducers are specified atop the fitted curves. The final selected Construct is shown boxed. Each data point represent mean and s.d. from four independent colonies.
Source data
Extended Data Fig. 5 Details of characterization and Dose Responses experiments across iterations of Bactoneuron 7.
AS7A, AS7B, AS7C and AS7D; yielding corresponding parameter values (shown in figure) of Fold Change (F.C.), Σ Leakage (ΣL), Signal variation (S.V.), Bias (BAS#), Weights (WInducer) and Inducer Saturation points (Sat.). The fluorescence readings obtained from the dose response experiments were fitted with the Log-Sigmoid function (see Methods) to derive the Weight and Bias parameters. The induction states of the two constant inducers are specified atop the fitted curves. The final selected Construct is shown boxed. Each data point represent mean and s.d. from four independent colonies.
Source data
Extended Data Fig. 6 Detailed genetic circuit designs and plasmid maps of Bactoneurons that went through no iteration and were selected as final.
(a) BNeu AS1, (b) BNeu AS2, (c) BNeu AS3, (d) BNeu AS4, (e) BNeu AS8, (f) BNeu AS9. Names of plasmids constructed and incorporated in the circuits are also shown within the plasmid schematic. MCS represents empty network brick cloning site. ‘F’ and ‘B’ denotes forward and reverse direction of cassette respectively. The E2-crimson output of AS1 is altered with EGFP to give AS1* and EGFP output of AS2 is altered with E2-Crimson to give AS2*.
Extended Data Fig. 7 Detailed genetic circuit designs and plasmid maps of Bactoneuron 5 across iterations.
(a) BNeu AS5A, (b) BNeu AS5B, (c) BNeu AS5C, (d) BNeu AS5D, (e) BNeu AS5E, (f) BNeu AS5F and (g) BNeu AS5G. Names of plasmids constructed and incorporated in the circuits are also shown within the plasmid schematic. MCS represents empty network brick cloning site. ‘F’ and ‘B’ denotes forward and reverse direction of cassette respectively.
Extended Data Fig. 8 Detailed genetic circuit designs and plasmid maps of Bactoneuron 6 and Bactoneuron 7 across iterations.
(a) BNeu AS6A, (b) BNeu AS6B, (c) BNeu AS6C, (d) BNeu AS6D, (e) BNeu AS6E, (f) BNeu AS6F, (g) BNeu AS7A, (h) BNeu AS7B, (i) BNeu AS7C and (j) BNeu AS7D. Names of plasmids constructed and incorporated in the circuits are also shown within the plasmid schematic. MCS represents empty network brick cloning site. ‘F’ and ‘B’ denotes forward and reverse direction of cassette respectively.
Extended Data Fig. 9 Detailed genetic circuit designs and plasmid maps of Bactoneurons.
(a) BNeu AS10, (b) BNeu AS11 and AS11*, (c) BNeu AS12, (d) BNeu AS13, (e) BNeu AS14 and AS14*. Names of plasmids constructed and incorporated in the circuits are also shown within the plasmid schematic. MCS represents empty network brick cloning site. ‘F’ and ‘B’ denotes forward and reverse direction of cassette respectively. The EGFP output of AS11 is altered with mTagBFP2 to give AS11* and EGFP output of AS14 is altered with mKO2 to give AS14*.
Extended Data Fig. 10 Experimental Characterizations of Bactoneurons.
Fold change characterization, Dose Responses experiments with fitting values, simulation, and re-validation of Bactoneurons AS10, AS11, AS12, AS13, and AS14. Each data point represent mean and s.d. from four independent colonies.
Source data
Supplementary information
Supplementary Information
Supplementary Fig. 1 and Tables 1–5.
Reporting Summary
Source data
Source Data Fig. 2
Statistical source data for threshold parameters obtained from Extended Data Figs. 2–5.
Source Data Fig. 3
Statistical source data for simulation and validation of bactoneurons.
Source Data Extended Data Fig. 2
Statistical source data for characterization and dose–response of bactoneurons.
Source Data Extended Data Fig. 3
Statistical source data for characterization and dose–response of bactoneurons.
Source Data Extended Data Fig. 4
Statistical source data for characterization and dose–response of bactoneurons.
Source Data Extended Data Fig. 5
Statistical source data for characterization and dose–response of bactoneurons.
Source Data Extended Data Fig. 10
Statistical source data for characterization, dose–response, simulation and experimental validation of bactoneurons.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Cite this article
Bonnerjee, D., Chakraborty, S., Mukherjee, B. et al. Multicellular artificial neural network-type architectures demonstrate computational problem solving.
Nat Chem Biol (2024). https://doi.org/10.1038/s41589-024-01711-4
-
Received: 23 June 2023
-
Accepted: 26 July 2024
-
Published: 16 September 2024
-
DOI: https://doi.org/10.1038/s41589-024-01711-4