8 photos
However promising the hydrogen was on paper, it also has many drawbacks. Even though it is arguably the most abundant element on Earth, it’s almost impossible to find it in a pure state. It is mostly part of other chemical elements, the most common being water and fossil fuels. These are also the easiest to extract hydrogen from, even though it’s not easy at all.
In fact, it takes a lot of energy to break these molecules apart and release the hydrogen. Water is cleaner, but also more energy consuming and thus more expensive. The hydrogen from water is considered “green” because it doesn’t have harmful by-products. When you extract hydrogen from fossil fuel, you must find a solution for the carbon, which is most often released as carbon dioxide. If you recover and store it, you get almost clean hydrogen, also called blue hydrogen. If carbon dioxide is released into the atmosphere, well, it’s bad for the environment.
How you get the hydrogen doesn’t matter very much because there are bigger problems than energy waste and pollution. Storing and transporting it to where it is needed is an even bigger challenge, as hydrogen is highly volatile and flammable. The best storage method to pack the most hydrogen in the smallest volume is by liquifying it. This is achieved when the gas is cooled to extremely low temperatures, below minus 253 degrees Celsius (-423 Fahrenheit). The advantage is that you don’t need high-pressure reservoirs to store it that way, but reaching that low temperature presents new challenges.
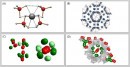
A team of researchers from the Ulsan National Institute of Science and Technology (UNIST) in Korea has discovered a new storage technology. The research centers around a nanoporous magnesium borohydride structure (Mg(BH₄)₂) that can store hydrogen at high densities even under normal atmospheric pressure. The nanoporous complex hydride comprising magnesium hydride, solid boron hydride (BH₄)₂), and magnesium cation (Mg+) enables the storage of five hydrogen molecules in a three-dimensional arrangement.
This allows an impressive hydrogen storage capacity of 144 g/L per volume of pores, surpassing traditional methods, such as storing hydrogen as a gas in a liquid state (70.8 g/L). This represents a paradigm shift in hydrogen storage, offering a compelling alternative to traditional, more expensive methods. The discovery paves the way for large-scale hydrogen storage systems for public transportation applications.
With an efficient and affordable storage system, hydrogen can re-emerge as a viable alternative to fossil fuels. Perhaps less so for passenger cars, considering that battery-electric vehicles are already dominating the market. However, hydrogen remains a great solution for heavy-duty applications and other transportation sectors, such as shipping and aviation.