Abstract
Necrotizing enterocolitis (NEC) represents a severe condition in infants, with perforation being a particularly critical pathological manifestation. However, there is an absence of guidelines regarding the refeeding of infants recovering from perforation subsequent to NEC. This study aimed to determine the optimal refeeding method for term infants recovering from perforation after NEC. The study encompassed three aspects: the timing of enteral nutrition (EN) resumption, the progression of EN, and the method of EN resumption. Ninety full-term neonates who developed perforation following NEC and underwent surgical intervention were included. These samples were divided into early enteral nutrition (EEN, < 7 days) and late enteral nutrition (LEN, ≥ 7 days) groups based on the timing of EN resumption; faster increase (FI, ≥ 20 ml/kg/d) and slower increase (SI, < 20 ml/kg/d) groups based on the progression of EN; intact protein formula (IPF), special medical formula (SMF, including EHF and AABF), and mixed feeding (MF) groups based on the method of EN resumption. EEN infants had a lower incidence of intestinal stenosis and reoperation (43.5% vs. 77.6%, p = 0.002; 60.9% vs. 82.1%, p = 0.038), and a shorter duration of hospital stay after surgery and parenteral nutrition (PN) than LEN infants (14 days vs. 20 days, p < 0.001; 11 days vs. 17 days, p < 0.001). Faster increasing feed volumes was associated with shorter duration of hospital stay and parenteral nutrition (15 days vs. 20 days, p < 0.001; 14 days vs. 17 days, p < 0.001), but a slower rate of weight gain (0.020 kg vs. 0.129 kg, p < 0.01). The time to repeat NPO in SMF group is shorter than IPF an MF groups (3 days vs. 4 days and 9 days, p = 0.025). Our study demonstrates the beneficial effects of early enteral feeding and fast advancement of feed volumes in term infants with NEC and perforation after surgery, specifically in reducing short-term complications and the duration of hospital stay following surgery and PN. Additionally, this study suggests that IPF and MF significantly contribute to stimulate intestinal adoption recovery.
Introduction
Necrotizing enterocolitis (NEC) is a devastating disease of newborns1, with a mortality rate of 10–45%2,3,4. Bell’s staging classifies the severity of NEC into three stages, where Stage III, termed “surgical NEC”, exhibits a higher mortality rate than Stages Iand II, referred to as “medical NEC”5,6,7. Intestinal perforation is a critical diagnostic indicator for stage III of NEC, necessitating urgent surgical intervention and nil per os (NPO) status4,6.
Infants with surgical NEC are at increased risk of complications, including parenteral nutrition-associated liver dysfunction (PNALD) and catheter-related sepsis due to prolonged use of parenteral nutrition and central venous catheters8,9,10,11,12,13,14. Intestinal stricture is a common postoperative complication, occurring in 9–36% of NEC patients15,16,17. Recent studies have suggested that initiating enteral feeding within seven days of necrotizing enterocolitis may contribute to reducing the incidence of intestinal stenosis18. Consequently, the development of rational refeeding guidelines for neonates with NEC following perforation is of paramount importance. While some research has demonstrated that standardized feeding protocols can decrease the occurrence of NEC19,20, there remains a lack of specific criteria for post-NEC infants21.
Although NEC is predominantly observed in premature infants, it can also affect term infants4,22,23,24. While previous studies have predominantly focused on resuming enteral nutrition in preterm infants after NEC, there remains a paucity of studies addressing term infants, particularly those with NEC complicated by perforation. This study aims to evaluate the timing for restarting feeds, the progression of enteral feeding, and the type of enteral nutrition in term infants who have undergone intestinal perforation subsequent to NEC. The findings of this study may provide valuable guidance on feeding strategies for infants recovering from perforation after NEC.
Methods
Study population
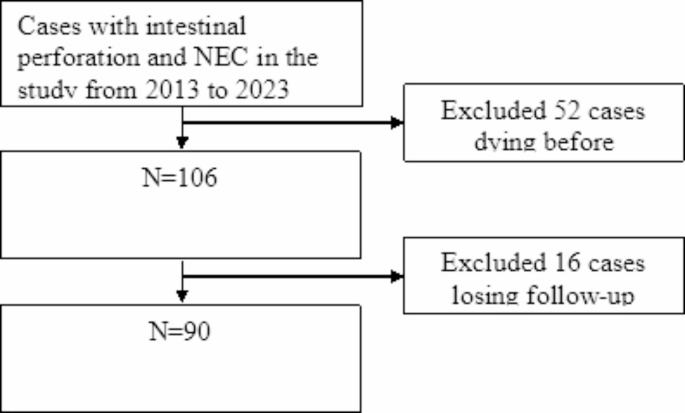
The retrospective analysis included data from term infants with intestinal perforation and NEC who received treatment at the NICU of Chongqing Medical University from May 2013 to May 2023. All patients were diagnosed with NEC by intraoperative findings (intestinal ischemia, necrosis, perforation, and bacterial slime adherence) and pathological examination (mucosal edema, hemorrhage and transmural necrosis), and perforations were confirmed by laparotomy, while cases involving neonates who passed away before refeeding or were lost to follow-up were excluded. A total of 90 cases met the inclusion criteria for this study. The distribution of study subjects and the reasons for exclusion are detailed in Fig. 1. Consent to participate was waived by the Ethics Committee of Children’s Hospital Affiliated to Chongqing Medical University.

Flow chart of the study.
Timing of enteral nutrition resumption
The patients were divided into two groups based on the timing of enteral feeding resumption: early enteral feeding (EEN, < 7 days) and late enteral feeding (LEN, ≥ 7 days). A previous study, which reintroduced enteral nutrition after excluding portal venous gas (PVG) on abdominal ultrasound for 3 days, reported an average reinitiation time of 4 days (range 3–6) for surgically treated NEC neonates2. An international survey on post-NEC patient management revealed that approximately 46% of surgeons fasted postoperative infants for 5–7 days, 42% for more than 7 days, and 12% for less than 5 days1. Therefore, a 7-day cutoff was established for this study.
Progression of enteral nutrition
Four data points were excluded due to re-fasting within 24 h after resuming feeding. The remaining 86 data points were divided into two groups: faster increase (FI, ≥ 20 ml/kg/day) and slower increase (SI, < 20 ml/kg/day). Existing research indicates that there is no significant increase in adverse effects or NEC recurrence with feeding advancement rates of 10–24 ml/kg/day2,12,25–28. Five of these studies involved Bell stage II, with one focusing on neonates undergoing gastrointestinal surgery (maximum advancement rate of 24 ml/kg/d)28. Consequently, a cutoff of 20 ml/kg/day was selected for this analysis.
Method of enteral nutrition resumption
In our institution, neonatologists identified four categories of enteral nutrition for resuming feeding in NEC infants: conventional term formula, extensively hydrolyzed formula (EHF), amino acid-based formulas (AABF), and mixed feeding. Due to the limited number of patients receiving AABF (n = 1), this group was merged with the EHF category. The patients were then divided into three groups: intact protein formula (IPF), special medical formula (SMF, including EHF and AABF), and mixed feeding (MF).
Statistical analysis
Counts/percentages were used for categorical variables and medians (interquartile ranges, IQRs) or means ± standard deviations (SD) for continuous variables while describing demographic and clinical data. 1 data (1.1%, <5%) about the cesarean section was missed, and we did not perform any treatment during the analysis; instead, we simply did not analyze this portion of the missing data. 6 data (6.7%) about the type of nutrition before NEC was missed, we applied Multiple Imputation to handle this portion of the missing data. For analyzing “time to restart NE” and “advancement of EN”, data were compared between two groups using Student’s t-test or Mann-Whitney U test for continuous variables, the Chi-squared test, Yates’s correction for continuity or the Fisher’s exact test for categorical indicators. For analyzing “type of restarting EN”, dates were compared between three groups using Kruskal-Wallis test for continuous variables, the Chi-squared test or the Fisher’s exact test for categorical indicators. Bonferroni correction was used for the post hoc test. The statistical analyses were conducted using SPSS 27.0 for Windows (IBM Corp., Armonk, NY, USA), and a p-value < 0.05 was considered significant.
Results
42cases underwent intestinal resection and enterostomy, 1 case underwent intestinal resection and anastomosis, 43 patients underwent repair of bowel perforation and enterostomy, 4 patients received enterostomy alone. Comorbidities were diagnosed through surgical and pathological examination. Fortunately, no neonates experienced catheter-related sepsis, short bowel syndrome, stoma prolapse, NEC relapse, or mortality. Notably, none of the patients received extensively hydrolyzed formula (EHF) or amino acid-based formulas (AABF) before developing NEC.
Timing of enteral nutrition resumption
The early enteral nutrition (EEN) group resumed enteral feeding at a median of 5 days (IQR 4–5), whereas the late enteral nutrition (LEN) group restarted feeding at a median of 9 days (IQR 8–11). Despite some missing data, most demographic and clinical characteristics between the EEN and LEN groups were comparable. The two groups showed a statistically significant difference in the type of nutrition prior to the development of NEC (p = 0.038). The Bonferroni correction revealed that the significant difference is primarily attributed to the rate of human milk feeding between the two groups. Infants in the EEN group exhibited a higher incidence of exclusive human milk (HM) feeding before NEC compared to the LEN group (39.1% vs. 14.9%), see Table 1.
Though there was no statistical difference in surgical procedure between the two groups (p = 0.051), the proportion of patients in the EEN group underwent bowel perforation repair with enterostomy was higher than that in LEN group (65.2% vs. 41.8%), and the proportion of patients who underwent bowel resection with enterostomy was lower than that in LEN group (26.1% vs. 53.7).
The outcomes are presented in Table 2. Primary outcomes revealed a lower incidence of intestinal stenosis in the EEN group compared to the LEN group (43.5% vs. 77.6%, p = 0.002). Two infants in the LEN group developed PNALD (3.0%), and one infant developed stoma necrosis (1.5%), while no such cases were observed in the EEN group; however, the difference did not achieve statistical significance (p = 1.000). Additionally, the EEN group exhibited a lower reoperation rate (60.9% vs. 82.1%, p = 0.038), as well as significantly reduced length of postoperative stay (LOPS) and duration of parenteral nutrition (PN) (14 days vs. 20 days, p < 0.001; 11 days vs. 17 days, p < 0.001). However, there was no statistically significant difference in the rate of repeated nil per os (NPO) or hospital infections between the two groups. Furthermore, there was no significant variation in total and daily weight gain after surgery between the EEN and LEN groups.
Progression of enteral nutrition
The rate of feeding advancement for the FI group was 22.4 (20.5–26.1) ml/kg/d, while that for the SI group was 14.2 (12.0-16.2) ml/kg/d. Table 3 presents the primary demographic and clinical characteristics of the patients. In the SI group, data on the feeding type before NEC for 6 patients were absent due to incomplete medical records. The FI group exhibited a lower average gestational age and birth weight (38.7 ± 0.9 vs. 39.2 ± 1.2, p = 0.006) and birth weight (2982 ± 530 g vs. 3341 ± 530 g, p = 0.014) compared to the SI group. The results indicated a higher prevalence of human milk (HM) and mixed feeding prior to NEC in the FI group, although these differences were not statistically significant.
Further insights into refeeding conditions, specifically the time taken to resume feeding and the method of reintroducing enteral nutrition, are outlined in Table 3. The FI group demonstrated a shorter median time to recommence feeding compared to the SI group (7 days vs. 9 days, p = 0.04). Additionally, the use of IPF during refeeding was significantly higher in the FI group than in the SI group (11.8% vs. 0.0%, p = 0.037).
The primary outcomes are presented in Table 4. No significant difference in PNALD or stoma necrosis were observed between the FI and SI groups. However, the FI group exhibited a lower incidence of intestinal stenosis (58.8% vs. 72.5%, p = 0.273), shorter postoperative stay (15 vs. 20 days, p < 0.001) and duration of parenteral nutrition (14 vs.17 days, p < 0.001) compared to the SI group. Although the daily weight gain in the FI group was lower than that in the SI group (0.020 vs. 0,129 kg, p = 0.01), no significant difference in total weight gain was observed. Repeated NPO rates and hospital infections were similar between the groups.
Method of enteral nutrition resumption
The main demographic and clinical data of the patients are summarized in Table 5. Due to incomplete medical records, the mode of delivery for one case in the Mixed Feeding (MF) group and the feeding methods for three cases in both of the Special Medical Formula (SMF) and MF groups were unavailable. Overall, most characteristics of the Early Enteral Nutrition (EEN) and Late Enteral Nutrition (LEN) groups were similar. The IPF group demonstrated the quickest speed of advancement (23.6 ml/lg/d), followed by the MF group second (16.0 ml/kg/d), with the SMF group being the slowest, these differences were statistically significance (p = 0.024).
Upon comparison of stoma location, a statistically significant difference was observed among the three groups (p = 0.012). All patients in the IPF group (n = 2, 100.0%) received transverse colostomy, 10 (16.9%) cases in the SMF group and 2 (7.1%) cases in the MF group. The Bonferroni correction revealed that the significant difference primarily arose between the IPF group and the SMF group, as well as between the IPF group and the MF group, with no difference observed between the SMF group and the MF group. The detailed length of the stoma proximal small bowel was not documented for 26 (44.1%) cases in the SMF group and 11 (39.2%) cases in the MF group, however, these patients underwent terminal ileostomy, which transect the small bowel within 15 centimeters from the ileocecal junction.
The outcomes were presented in Table 6. In the MF group, two cases of Parenteral Nutrition-Associated Liver Disease (PNALD) were observed, while one case of stoma necrosis was reported in the SMF group. These differences were not statistically significant (p = 0.139 and p = 1.000, respectively). The incidence of intestinal stenosis was associated with the type of refeeding, with rates of 0.0% in the IPF group, 65% in the SMF group, and 82.1% in the MF group (p = 0.03). Correction for multiple comparisons using the Bonferroni method indicated significant differences between the IPF group versus the SMF and MF groups. No statistically significant differences were observed in the reoperation rate among the IPF, SMF, and MF groups (p = 0.066). Notably, the MF group exhibited a higher reoperation rate compared to the IPF and SMF groups (89.3% vs. 50% and 70%, respectively).
No significant differences were observed among the three groups regarding Length of Postoperative Stay (LOPS) and Parenteral Nutrition (PN) duration (p = 0.152 and p = 0.385, respectively). Although the daily weight gain post operation showed no statistically significant difference, the IPF group exhibited higher weight gain compared to the SMF and MF groups (0.035 kg/d in IPF group vs. 0.016 kg/d in SMF group vs. 0.015 kg/d in MF group, p = 0.057). Additionally, there was no variance in total weight gain among the three groups. The findings also revealed no significant difference in the incidence of repeating NPO between the groups (p = 0.265). Nonetheless, the time to repeat NPO in the MF group was notably longer than that in the other two groups (9 days vs. 4 days in IPF group vs. 3 days in SMF group, p = 0.025). Additionally, no statistically significant differences were observed in the incidence of hospital infection among the groups (p = 0.756). Further post hoc testing revealed no statistically differences in pairwise comparisons of the hospital infection rate.
Discussion
Prolonged use of Enteral Nutrition (EN) is closely associated with the development of Parenteral Nutrition-Associated Liver Disease (PNALD), catheter-related sepsis, and other complications. The process of restarting feeding constitutes a critical component of postoperative NEC management. Nevertheless, there is a significant absence of feeding guidelines for patients who have undergone surgery for NEC and perforation. Our retrospective study analyzed 90 patients diagnosed with perforation and NEC at Children’s Hospital of Chongqing Medical University over the past few decades.
Time to restart feeding
Clinicians frequently opt to recommence feeding at least 7 days after a diagnosis of NEC29. An international investigation showed that 46% surgeons kept NPO for 5 to 7 days after surgery of NEC, 42% abstained > 7 days, and 12% less than 5 days1.
Frantz et al.30 reported clinical and radiological relapses in several NEC patients who recommenced feeding within 10 days, although specific patient details were not disclosed in the study. It seems that prolonged NPO duration after NEC may be safe for neonates. However, prolonged NPO duration has been associated with morphology atrophy, decreased villous height, increased mucosal permeability, compromised gut-associated lymphoid tissue (GALT) function, diminished intestinal IgA production, weakened mucosal barrier function, and eventual translocation of intestinal bacteria31,32,33,34. A study by Aileen et al.35 suggested that insufficient enteral nutrition was associated with bacterial overgrowth, attenuated mucosal inflammatory response, potential intestinal bacterial translocation, and subsequent sepsis.
Early Enteral Nutrition (EEN) has shown benefits for both adult and infant populations following abdominal surgery. Research in adults undergoing gastrointestinal surgery demonstrated that EEN could reduce infectious complications, hospitalization duration, PNALD, anastomotic leak, and dehiscence36,37,38,39. EEN has also been shown to be beneficial for neonates by promoting intestinal adaptation, reducing length of stay, time to full enteral nutrition, complications (PNALD, intestinal obstruction, sepsis), and feeding intolerance9,40,41,42,43,44,45.
EEN post-NEC surgery could serve as a protective strategy, facilitating the recovery of intestinal injury without increasing complications. Studies by Bohnhorst et al.2, Brotschi et al.12 and Hock et al.46 indicated that EEN did not elevate the risk of bowel obstruction, stenosis post-NEC, NEC relapse, gastrointestinal bleeding, abdominal distension, or high gastric residuals volume in preterm infants at stages Bell II or III. Additionally, EEN decreased central venous catheterization duration and catheter-related sepsis incidence. An animal study demonstrated that reduced enteral nutrition led to a decrease in intestinal epithelial cell production and villus height, impairing intestinal adaptation34.
Early feeding following gastrointestinal anastomosis was not associated with delayed healing of the anastomosis or an increased incidence of leakage47. A multicenter controlled trial involving neonates with congenital gastrointestinal issues undergoing intestinal anastomosis showed that feeding within 48 h post-operation did not decrease length of postoperative stay (LOPS), nor did it increase the incidence of gastrointestinal complications. Therefore, EEN in neonates following abdominal surgery is considered safe and may potentially reduce costs due to a shorter length of stay.
Our study found that reinitiating feeding for term infants within 7 days following NEC and perforation surgery significantly reduced the incidence of intestinal stenosis and reoperation rates, as well as shortened LOPS and PN duration, consistent with previous research findings.
Progression of enteral nutrition
Current guidelines lack of a standardized protocol regarding the rate of advancing Enteral Nutrition (EN) after surgery for Necrotizing Enterocolitis (NEC)21. A Cochrane review from 2021 indicated that advancing enteral feeding slowly (up to 24 ml/kg/d) did not reduce the incidence of NEC or mortality in very low birth weight infants, but may increase the risk of infection48. Therefore, we hypothesized that a more rapid advancement of EN might not increase the risk of NEC relapse or other complications. Our findings support this hypothesis, demonstrating that a rapid increase in EN (over 20 ml/kg/d) was not associated with a higher incidence of complications, and no instances of NEC relapse were observed.
Prior studies have demonstrated that reintroducing feeding to neonates following gastrointestinal surgery with an advancement in feeding volumes of 20 ml/kg/d did not increase the risk of complications such as catheter-related sepsis or intestinal strictures. This method also reduced the duration of central venous catheter use in both term and preterm infants with NEC (Bell stage II and III)2,12. Additionally, infants undergoing gastrointestinal surgery for various reasons were able to tolerate advancing feeds by 15–20 ml/kg every 12–24 h or 24 ml/kg/d, resulting in a reduced incidence and severity of Parenteral Nutrition-Associated Liver Disease (PNALD)26,27,28. The more rapid advancement of feeding contributes to the early cessation of PN.
Method of enteral nutrition resumption
Human milk remains the preferred nutrition source for infants during the refeeding process post-surgery44,49. Breast milk is rich in components including secretory IgA (sIgA), lysozyme, oligosaccharides, growth factors, nitric oxide precursor molecules, and bifidobacterial, all of which play essential roles in immunological defenses and the functional adaptation of the intestines50,51,52,53,54,55. Despite the numerous benefits of human milk, some infants may receive formula or a combination of human and formula milk due to factors such as limited availability of donor milk, inadequate breast milk supply, or other social considerations56. It is noteworthy that none of the 90 infants in our study received human milk. Research on mixed feeding has suggested that a higher intake of human milk is associated with improved weight gain and healing of Necrotizing Enterocolitis (NEC)57.
When human milk is unavailable, Extensively Hydrolyzed Formula (EHF) is recommended as the primary option for infants with intestinal conditions29,44,58. A nationwide survey indicated that the primary reason for EHF utilization is recovery from NEC stages II or III59. Infants recovering from intestinal mucosal inflammation and gastrointestinal surgery may be at increased risk for an antigen response to intact protein and cow’s milk protein allergy (CMPA)25,60,61. CMPA should be considered in cases of NEC recurrence or onset where no typical risk factors are identified, and the use of EHF or Amino Acid-Based Formula (AABF) could help prevent NEC relapse in infants at risk of CMPA25,62. Consequently, EHF or AABF was commonly used in neonates recovering from NEC.
It is noteworthy that there is debate regarding the protein absorption capacities in the intestines of neonates following intestinal operations. While some studies indicate that infants can absorb 70-90% of protein intake, others have shown no significant difference in mucosal hyperplasia between hydrolyzed and non-hydrolyzed protein formulas63,64. The choice between EHF and Intact Protein Formula (IPF) has shown varying impacts on weight gain, with certain studies reporting similar weight gain velocities among healthy term infants and those with short bowel syndrome65,66,67. However, some studies have suggested slower weight gain with EHF compared to IPF in piglets with short bowel syndrome and preterm infants, potentially leading to less efficient nutrition absorption and slower weight gain29,68,69.
Intestinal resection can lead to increased intestinal peristalsis and disrupted bile acid secretion, affecting fat absorption29. Medium-chain triglycerides (MCTs) are more readily absorbed by infants post intestinal resection as their assimilation does not require bile acid involvement70. Moreover, lactose intolerance is regarded as a potential risk factor for NEC, particularly in infants with decreased lactase production due to a reduced mucosal surface area following intestinal resection29,71. The majority of EHF products are lactose-free29,59, which is advantageous for facilitating intestinal absorption and functional adaptation following gastrointestinal surgery. However, considering that human milk contains lactose and remains the preferred option post NEC, there is no compelling evidence to exclude lactose.
The inclusion of a diverse range of nutrients may facilitate the restoration of intestinal function in infants recovering from NEC. Researches have shown that polymeric feeds could contribute to enhanced regeneration of intestinal mucosa in rats undergoing intestinal resection, whereas MCTs were found to not stimulate mucosal adaptation to the same extent as long-chain triglycerides (LCTs) in a separate study72,73.
Despite the widespread use and established safety of EHF in clinical settings, definitive evidence regarding the optimal feeding strategy for infants with NEC is still lacking. Our study found no significant differences in the incidence of Parenteral Nutrition-Associated Liver Disease (PNALD), stoma necrosis, bowel obstruction, or reoperation among infants receiving IPF, special medical formula (EHF and AABF), or a combination thereof. The intact protein formula was associated with a lower incidence of intestinal stenosis, which potentially attribute to the small sample size of the IPF group, while no significant differences were observed between the special medical formula and mixed feeding groups. Additionally, despite the absence of statistical differences in the rate of returning to Nil Per Os (NPO) due to severe feeding intolerance among infants fed IPF, SMF, or mixed feeding, the mixed feeding group exhibited the longest time to return to NPO, followed by the IPF group, and subsequently the SMF group. The complexity of nutrients is pivotal in stimulating intestinal adaptation following NEC and perforation surgery, as breast milk provides crucial protective benefits against feeding intolerance post-intestinal resection. Although short-term total weight gain after surgery was comparable across the three feeding groups, the IPF group exhibited superior daily weight gain.
Conclusions
Re-feeding infants within 7 days following surgery for NEC and perforation has been demonstrated to reduce short-term complications including PNALD, stoma necrosis, bowel obstruction, and stenosis, as well as to decrease the rate of reoperation.
Advancing feeding volumes by at least 20 ml/kg/d does not appear to be associated with a higher incidence of complications or reoperation. Both early eternal feeding and rapid advancement of volumes are associated with shorter hospital stay durations post operation and durations of PN.
No significant difference in total weight gain was observed among infants receiving IPF, EHF/AABF or mixed feeding, but IPF feeding was associated with a more rapid daily weight gain following surgery. Although both of these three feeding methods demonstrated similar rates of complications and reoperation, IPF and mixed feeding appeared to play a more significant role in promoting intestinal recovery. Refeeding with IPF and mixed feeds was deemed safe and beneficial for restoring intestinal function while closely monitoring abdominal signs. It is important to note that due to the retrospective nature of the study, the sample size of the IPF group was limited. Future prospective studies with larger sample sizes are necessary to validate the safety and efficacy of IPF in infants with NEC.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
-
Zani, A. et al. International survey on the management of necrotizing enterocolitis. Eur. J. Pediatr. Surg. Off J. Austrian Assoc. Pediatr. Surg. Al Z. Kinderchir. 25 (1), 27–33 (2015).
Google Scholar
-
Bohnhorst, B. et al. Early feeding after necrotizing enterocolitis in preterm infants. J. Pediatr. 143 (4), 484–487 (2003).
Google Scholar
-
Han, S. M. et al. Trends in incidence and outcomes of necrotizing enterocolitis over the last 12 years: a multicenter cohort analysis. J. Pediatr. Surg. 55 (6), 998–1001 (2020).
Google Scholar
-
Neu, J. & Walker, W. A. Necrotizing enterocolitis. N Engl. J. Med. 364 (3), 255–264 (2011).
Google Scholar
-
Ou, J., Courtney, C. M., Steinberger, A. E., Tecos, M. E. & Warner, B. W. Nutrition in Necrotizing enterocolitis and following intestinal resection. Nutrients 12 (2), 520 (2020).
Google Scholar
-
Teresa, C., Antonella, D. & De Ville De Goyet Jean. New Nutritional and therapeutical strategies of NEC. Curr. Pediatr. Rev. 15 (2), 92–105 (2019).
Google Scholar
-
Schnabl, K. L., Aerde, J. E. V., Thomson, A. B. & Clandinin, M. T. Necrotizing enterocolitis: a multifactorial disease with no cure. World J. Gastroenterol. WJG. 14 (14), 2142–2161 (2008).
Google Scholar
-
Niccum, M., Khan, M. N., Middleton, J. P., Vergales, B. D. & Syed, S. Cholestasis affects enteral tolerance and prospective weight gain in the NICU. Clin. Nutr. ESPEN. 30, 119–125 (2019).
Google Scholar
-
Orso, G. et al. Pediatric parenteral nutrition-associated liver disease and cholestasis: novel advances in pathomechanisms-based prevention and treatment. Dig. Liver Dis. 48 (3), 215–222 (2016).
Google Scholar
-
Postuma, R. & Trevenen, C. L. Liver disease in infants receiving total parenteral nutrition. Pediatrics 63 (1), 110–115 (1979).
Google Scholar
-
Stoll, B. J. et al. Late-Onset Sepsis in very low Birth Weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics 110 (2), 285–291 (2002).
Google Scholar
-
Brotschi, B., Baenziger, O., Frey, B., Bucher, H. U. & Ersch, J. Early enteral feeding in conservatively managed stage II necrotizing enterocolitis is associated with a reduced risk of catheter-related sepsis. jpme 37 (6), 701–705 (2009).
Google Scholar
-
Odetola, F. O., Moler, F. W., Dechert, R. E., VanDerElzen, K. & Chenoweth, C. Nosocomial catheter-related bloodstream infections in a pediatric intensive care unit: risk and rates associated with various intravascular technologies. Pediatr. Crit. Care Med. J. Soc. Crit. Care Med. World Fed. Pediatr. Intensive Crit. Care Soc. 4 (4), 432–436 (2003).
-
Sohn, A. H. et al. Prevalence of nosocomial infections in neonatal intensive care unit patients: results from the first national point-prevalence survey. J. Pediatr. 139 (6), 821–827 (2001).
Google Scholar
-
Horwitz, J. R. et al. Complications after surgical intervention for necrotizing enterocolitis: a multicenter review. J. Pediatr. Surg. 30 (7), 994–998 (1995). discussion 998–999.
Google Scholar
-
Kosloske, A. M., Burstein, J. & Bartow, S. A. Intestinal obstruction due to colonic stricture following neonatal necrotizing enterocolitis. Ann. Surg. 192 (2), 202–207 (1980).
Google Scholar
-
Bütter, A., Flageole, H. & Laberge, J. M. The changing face of surgical indications for necrotizing enterocolitis. J. Pediatr. Surg. 37 (3), 496–499 (2002).
Google Scholar
-
Patel, E. U., Wilson, D. A., Brennan, E. A., Lesher, A. P. & Ryan, R. M. Earlier re-initiation of enteral feeding after necrotizing enterocolitis decreases recurrence or stricture: a systematic review and meta-analysis. J. Perinatol. 40 (11), 1679–1687 (2020).
Google Scholar
-
Fu, T. T. et al. Standardizing Feeding Strategies in Moderately Preterm Infants. Res Sq. Published online February 6, 2023:rs.3.rs-2520889.
-
Kamitsuka, M. D., Horton, M. K. & Williams, M. A. The incidence of necrotizing enterocolitis after introducing standardized feeding schedules for infants between 1250 and 2500 grams and less than 35 weeks of gestation. Pediatrics 105 (2), 379–384 (2000).
Google Scholar
-
Perks, P. & Abad-Jorge, A. Nutritional Management of the Infant with Necrotizing Enterocolitis.
-
Martinez-Tallo, E., Claure, N. & Bancalari, E. Necrotizing enterocolitis in full-term or near-term infants: risk factors. Biol. Neonate. 71 (5), 292–298 (1997).
Google Scholar
-
Wiswell, T. E., Robertson, C. F., Jones, T. A. & Tuttle, D. J. Necrotizing enterocolitis in full-term infants. A case-control study. Am. J. Dis. Child. 1960. 142 (5), 532–535 (1988).
Google Scholar
-
Raboei, E. H. Necrotizing enterocolitis in full-term neonates: is it aganglionosis? Eur. J. Pediatr. Surg. Off J. Austrian Assoc. Pediatr. Surg. Al Z. Kinderchir. 19 (2), 101–104 (2009).
Google Scholar
-
Christian, V. J., Polzin, E. & Welak, S. Nutrition Management of Necrotizing enterocolitis. Nutr. Clin. Pract. 33 (4), 476–482 (2018).
Google Scholar
-
Shores, D. R. et al. Implementation of feeding guidelines in infants at risk of intestinal failure. J. Perinatol. 35 (11), 941–948 (2015).
Google Scholar
-
Shores, D. R. et al. Post-operative Enteral Nutrition guidelines reduce the risk of intestinal failure-associated Liver Disease in Surgical infants. J. Pediatr. 195, 140–147e1 (2018).
Google Scholar
-
Savoie, K. B. et al. Standardization of feeding Advancement after neonatal gastrointestinal surgery: does it improve outcomes? Nutr. Clin. Pract. 31 (6), 810–818 (2016).
Google Scholar
-
Embleton, N. D. & Zalewski, S. P. How to feed a baby recovering from necrotising enterocolitis when maternal milk is not available. Arch. Dis. Child. – Fetal Neonatal Ed. 102 (6), F543–F546 (2017).
Google Scholar
-
Frantz, I. D., L’heureux, P., Engel, R. R. & Hunt, C. E. Necrotizing enterocolitis. J. Pediatr. 86 (2), 259–263 (1975).
Google Scholar
-
Welsh, F. et al. Gut barrier function in malnourished patients. Gut 42 (3), 396–401 (1998).
Google Scholar
-
Li, J. et al. Effects of parenteral and enteral nutrition on gut-associated lymphoid tissue. J. Trauma. 39 (1), 44–51 (1995). discussion 51–52.
Google Scholar
-
Heel, K. A., Kong, S. E., McCauley, R. D., Erber, W. N. & Hall, J. C. The effect of minimum luminal nutrition on mucosal cellularity and immunity of the gut. J. Gastroenterol. Hepatol. 13 (10), 1015–1019 (1998).
Google Scholar
-
Cronk, D. R., Ferguson, D. C. & Thompson, J. S. Malnutrition impairs postresection intestinal adaptation. J. Parenter. Enter. Nutr. 24 (2), 76–80 (2000).
Google Scholar
-
Smith, A. R. et al. Microbiological and immunological effects of enteral feeding on the upper gastrointestinal tract. J. Med. Microbiol. 60 (3), 359–365 (2011).
Google Scholar
-
Mazaki, T. & Ebisawa, K. Enteral versus Parenteral Nutrition after gastrointestinal surgery: a systematic review and Meta-analysis of Randomized controlled trials in the English literature. J. Gastrointest. Surg. 12 (4), 739–755 (2008).
Google Scholar
-
Gabor, S. et al. Early enteral feeding compared with parenteral nutrition after oesophageal or oesophagogastric resection and reconstruction. Br. J. Nutr. 93 (4), 509–513 (2005).
Google Scholar
-
Kudsk, K. A. et al. Enteral versus parenteral feeding. Effects on septic morbidity after blunt and penetrating abdominal trauma. Ann. Surg. 215 (5), 503–513 (1992).
Google Scholar
-
Lewis, S. J., Andersen, H. K. & Thomas, S. Early enteral nutrition within 24 h of intestinal surgery versus later commencement of feeding: a systematic review and meta-analysis. J. Gastrointest. Surg. Off J. Soc. Surg. Aliment. Tract. 13 (3), 569–575 (2009).
Google Scholar
-
Greer, D., Karunaratne, Y. G., Karpelowsky, J. & Adams, S. Early enteral feeding after pediatric abdominal surgery: a systematic review of the literature. J. Pediatr. Surg. 55 (7), 1180–1187 (2020).
Google Scholar
-
Ekingen, G., Ceran, C., Guvenc, B. H., Tuzlaci, A. & Kahraman, H. Early enteral feeding in newborn surgical patients. Nutrition 21 (2), 142–146 (2005).
Google Scholar
-
Jiang, W. W. et al. Early enteral nutrition for upper digestive tract malformation in neonates. Asia Pac. J. Clin. Nutr. 24(1), 38–42 (2015).
-
Jiang, W. W. et al. Early enteral nutrition in neonates with partial gastrectomy: a multi-center study. Asia Pac. J. Clin. Nutr. 25(1), 46–51 (2016).
-
Olieman, J. F. et al. Enteral Nutrition in children with short-bowel syndrome: current evidence and recommendations for the Clinician. J. Am. Diet. Assoc. 110 (3), 420–426 (2010).
Google Scholar
-
Beath, S. V. et al. Parenteral nutrition-related cholestasis in postsurgical neonates: multivariate analysis of risk factors. J. Pediatr. Surg. 31(4), 604–606 (1996).
-
Hock, A. M. et al. Initiation of Enteral Feeding after necrotizing enterocolitis. Eur. J. Pediatr. Surg. Off J. Austrian Assoc. Pediatr. Surg. Al Z. Kinderchir. 28 (1), 44–50 (2018).
Google Scholar
-
Tang, Z., Cai, H. & Cui, Y. Influence of early postoperative feeding in gastrointestinal anastomotic fistula formation and Healing Time in rabbits. BioMed. Res. Int. 2018, 1–6. https://doi.org/10.1155/2018/8258096 (2018).
Google Scholar
-
Oddie, S. J., Young, L. & McGuire, W. Slow advancement of enteral feed volumes to prevent necrotising enterocolitis in very low birth weight infants. Cochrane Database Syst. Rev. 8 (8), CD001241 (2021).
Google Scholar
-
Brindle, M. E. et al. Consensus guidelines for Perioperative Care in neonatal intestinal surgery: enhanced recovery after surgery (ERAS®) Society recommendations. World J. Surg.44 (8), 2482 (2020).
Google Scholar
-
Dallas, D. C., Underwood, M. A., Zivkovic, A. M. & German, J. B. Digestion of protein in premature and term infants. J. Nutr. Disord Ther. 2 (3), 112 (2012).
Google Scholar
-
Ramani, M. & Ambalavanan, N. Feeding practices and Necrotizing enterocolitis. Clin. Perinatol. 40 (1), 1–10 (2013).
Google Scholar
-
York, D. J., Smazal, A. L., Robinson, D. T. & De Plaen, I. G. Human milk growth factors and their role in NEC Prevention: a narrative review. Nutrients 13 (11), 3751 (2021).
Google Scholar
-
Elgin, T. G., Kern, S. L. & McElroy, S. J. Development of the neonatal intestinal Microbiome and its Association with Necrotizing enterocolitis. Clin. Ther. 38 (4), 706–715 (2016).
Google Scholar
-
Hackam, D. & Caplan, M. Necrotizing enterocolitis: pathophysiology from a historical context. Semin Pediatr. Surg. 27 (1), 11–18 (2018).
Google Scholar
-
Bering, S. B. Human milk oligosaccharides to prevent gut dysfunction and necrotizing enterocolitis in Preterm neonates. Nutrients 10 (10), 1461 (2018).
Google Scholar
-
Sisk, P. M., Lovelady, C. A., Dillard, R. G., Gruber, K. J. & O’Shea, T. M. Early human milk feeding is associated with a lower risk of necrotizing enterocolitis in very low birth weight infants. J. Perinatol. Off J. Calif. Perinat. Assoc. 27 (7), 428–433 (2007).
Google Scholar
-
Liu, K., Guo, J., Yang, J. & Su, Y. The Association of Human Milk Proportion with the clinical outcomes of necrotizing enterocolitis in Preterm infants: a retrospective study. Nutrients 15 (17), 3796 (2023).
Google Scholar
-
Goulet, O., Abi Nader, E., Pigneur, B. & Lambe, C. Short bowel syndrome as the Leading cause of Intestinal Failure in early life: some insights into the management. Pediatr. Gastroenterol. Hepatol. Nutr. 22 (4), 303 (2019).
Google Scholar
-
Lapillonne, A. et al. Use of extensively hydrolysed formula for refeeding neonates postnecrotising enterocolitis: a nationwide survey-based, cross-sectional study. BMJ Open. 6 (7), e008613 (2016).
Google Scholar
-
El Hassani, A. et al. Allergie aux protéines du lait de vache après chirurgie digestive néonatale. Arch. Pédiatrie. 12 (2), 134–139 (2005).
Google Scholar
-
Korai, T. et al. Neonates undergoing gastrointestinal surgery have a higher incidence of non-IgE-mediated gastrointestinal food allergies. Pediatr. Surg. Int. 34 (10), 1009–1017 (2018).
Google Scholar
-
Nehra, D., Fallon, E. M. & Puder, M. The Prevention and Treatment of Intestinal failure-associated liver disease in neonates and children. Surg. Clin. North. Am. 91 (3), 543–563 (2011).
Google Scholar
-
Schaart, M. W., De Bruijn, A. C. J. M., Tibboel, D., Renes, I. B. & Van Goudoever, J. B. Dietary protein absorption of the small intestine in human neonates. J. Parenter. Enter. Nutr. 31 (6), 482–486 (2007).
Google Scholar
-
Vanderhoof, J. A., Grandjean, C. J., Burkley, K. T. & Antonson, D. L. Effect of casein versus casein hydrolysate on mucosal adaptation following massive bowel resection in infant rats. J. Pediatr. Gastroenterol. Nutr. 3 (2), 262–267 (1984).
Google Scholar
-
Ksiazyk, J., Piena, M., Kierkus, J. & Lyszkowska, M. Hydrolyzed versus nonhydrolyzed protein diet in short bowel syndrome in children. J. Pediatr. Gastroenterol. Nutr. 35(5), 615–618 (2002).
-
Kantaras, P. et al. Growth and gut comfort of healthy term infants exclusively fed with a partially hydrolysed protein-based infant formula: a randomized controlled double-blind trial. Front. Pediatr. 12, 1328709 (2024).
Google Scholar
-
Fleddermann, M., Knoll, A. & Koletzko, B. Safety and Suitability of Infant Formula manufactured from extensively hydrolyzed whey protein compared to intact protein: a combined analysis of two Randomized Controlled studies. Nutrients 16 (2), 245 (2024).
Google Scholar
-
Ng, D. H. C., Klassen, J., Embleton, N. D. & McGuire, W. (eds) Protein hydrolysate versus standard formula for preterm infants. Cochrane Neonatal Group,. Cochrane Database Syst Rev. Published online October 2, (2017).
-
Bines, J. E. et al. Influence of diet complexity on intestinal adaptation following massive small bowel resection in a preclinical model. J. Gastroenterol. Hepatol. 17 (11), 1170–1179 (2002).
Google Scholar
-
Thureen, P. J. Jr WWH. Neonatal Nutrition and Metabolism, Second Edition.
-
Thymann, T. et al. Carbohydrate maldigestion induces necrotizing enterocolitis in preterm pigs. Am. J. Physiol. Gastrointest. Liver Physiol. 297 (6), G1115–1125 (2009).
Google Scholar
-
Lai, H. S., Chen, W. J., Chen, K. M. & Lee, Y. N. Effects of monomeric and polymeric diets on small intestine following massive resection. Taiwan. Yi Xue Hui Za Zhi. 88 (10), 982–988 (1989).
Google Scholar
-
Vanderhoof, J. A., Grandjean, C. J., Kaufman, S. S., Burkley, K. T. & Antonson, D. L. Effect of high percentage medium-chain triglyceride Diet on Mucosal Adaptation following massive bowel resection in rats. J. Parenter. Enter. Nutr. 8 (6), 685–689 (1984).
Google Scholar
Funding
This research was funded by Science- Health Joint Medical Scientific Research Project of Chongqing (2024MSXM119).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Children’s Hospital Affiliated to Chongqing Medical University (2024-79; 22 January 2024).
Author information
Authors and Affiliations
Contributions
Conceptualization, W.L., Y.H. and H.W.; methodology, W.L. and P.D.; formal analysis, W.L., S.H. and Z.L.; resources, W.L. and H.C.; investigation, W.L., Q.H. and J.Y.; writing—original draft preparation, W.L.; writing—review and editing, W.L. The first draft of the manuscript was written by W.L. and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
Reprints and permissions
About this article
Cite this article
Luo, W., Cao, H., Hu, Y. et al. Optimizing nutritional strategies in term NEC and perforation infants after intestinal operation: a retrospective study.
Sci Rep 15, 5577 (2025). https://doi.org/10.1038/s41598-025-90366-9
-
Received: 21 October 2024
-
Accepted: 12 February 2025
-
Published: 15 February 2025
-
DOI: https://doi.org/10.1038/s41598-025-90366-9
Keywords
- Term infants
- Necrotizing enterocolitis
- Intestinal perforation
- Intestinal resection
- Nutrition
- Intestinal stenosis