Abstract
Cancer is a leading cause of death globally. Immunotherapy has shown promise in treating various types of cancer, but its effectiveness varies among patients. The Controlling Nutritional Status (CONUT) score has been linked to the prognosis of different cancers. However, its predictive value for immunotherapy outcomes is not well understood. Our research represents the pioneering meta-study to examine the prognostic value of the CONUT score on cancer patients treated with an immune checkpoint inhibitor (ICI). A comprehensive literature search was conducted using various databases including PubMed, the Cochrane Library, EMBASE, and Google Scholar. The study was conducted until July 28, 2023. This analysis encompassed a comprehensive evaluation of various clinical outcomes, namely overall survival (OS), progression-free survival (PFS), objective response rate (ORR), and disease control rate (DCR). 663 patients from 8 studies were included in this study. It showed that cancer patients with high CONUT score had poorer OS (HR: 1.94, 95% CI, 1.52–2.47, p < 0.001) and PFS (HR: 2.22, 95% CI, 1.48–3.31, p < 0.001), as well as worse ORR (OR: 0.46, 95% CI, 0.25–0.85, p = 0.013) and DCR (HR: 0.29, 95% CI, 0.14–0.59, p = 0.001). The CONUT score can predict the prognosis of tumor patients treated with ICIs.
Introduction
One of the leading causes of death in the globe is cancer, only second to cardiovascular disease1,2. In just one year in 2020, 9.3 million new cancer cases were found around the globe, with 10 million deaths caused by cancer2. According to the GLOBOCAN statistics, the worldwide cancer burden is growing. Each year, 29.5 million new cancer diagnoses are projected by 2040, with 16.5 million cancer-related deaths (https://gco.iarc.fr/tomorrow), revealing a major burden on society and the economy. Great progress has been made in the treatment of cancer. Current therapeutic modalities for cancer management encompass a range of interventions, namely surgical resection, radiation therapy, chemotherapy, hormone therapy, Chinese medicine therapy, and immunotherapy3. Immune checkpoint inhibitors (ICI) therapy is a type of immunotherapy that targets drugs that inhibit PD1(Programmed Death-1), PD-L1(Programmed Death Ligand-1), and CTLA-4(Cytotoxic T Lymphocyte-associated Antigen-4) and has demonstrated encouraging outcomes in the treatment of a variety of tumors4,5, including non-small-cell lung cancer(NSCLC)6, renal cell carcinoma (RCC)7, hepatocellular carcinoma (HCC)8, and melanoma9. However, various individuals’ immune responses are variable, resulting in low immunological effectiveness in certain patients10. As a result, the development of robust biomarkers with high predictive value for assessing the prognosis of cancer patients following immunotherapy is of paramount importance, allowing for individualized and accurate immunotherapy.
It is generally understood that the patient’s immune nutritional condition is critical in cancer therapy11. Systemic inflammation and malnutrition are important prognostic indicators for malignant cancers12,13. Multiple nutritional assessment systems, including Nutritional Risk Screening (NRS), albumin (ALB), and prognostic nutritional index (PNI), have been shown to predict the prognosis of tumor patients14,15. The Controlling Nutritional Status (CONUT) score, is an innovative and straightforward clinical nutritional index, consisting of three blood measures: lymphocytes, albumin, and total cholesterol16. Ignacio et al. first introduced the CONUT score as a standard evaluation technique to evaluate the nutritional status of hospitalized patients 16 (The scoring criteria can be seen in Table 1). The CONUT scores are closely linked to the prognosis of diverse forms of malignancies, such as colorectal cancer, esophageal squamous cell carcinoma (ESCC), glioblastoma, gynecological cancer, pancreatic ductal adenocarcinoma, and gastric cancer (GC)17,18,19,20,21,22. Recently, CONUT has also been applied in predicting tumor immunotherapy. CONUT score was an independent predictor of the efficacy of treatment and OS in NSCLC23. However, no independent predictive effect was observed in gastric cancer24. To date, the prognostic value of novel inflammatory biomarker CONUT for ICIs is unknown in most tumor types, and no meta-analysis has been performed.
This research conducted a systematic evaluation of the predictive effect of CONUT in the treatment of cancers by ICIs.
Methods
Literature search strategies
This analysis was carried out with the PRISMA declaration25. On July 28, 2023, a thorough literature search was performed using several databases, such as PubMed, EMBASE, the Cochrane Library, and Google Scholar. Our search strategy was from the date of search construction to the date of search completion. Relevant papers were retrieved using several search phrases, encompassing MeSH terms and keywords, for example, “Immune Checkpoint Inhibitors [MeSH]”, “Checkpoint Blockade, Immune”, “Blockade, PD-1-PD-L1”, “CTLA-4 Inhibitors”, “Tislelizumab”, “Toripalimab”, “Envafolimab”, “Camrelizumab”, “Sintilimab”, “Nivolumab”, “Controlling Nutritional Status”, “CONUT”. Specific search strategies are available in the supplementary file. The search parameters have been restricted to the field of English literature only. A comprehensive elucidation of the search methodologies is presented in Table 2. In addition, a comprehensive search was conducted for grey literature utilizing the Google Scholar database. Grey literature was a Google preprint that failed to be published due to negative results. Furthermore, all qualified studies’ reference lists underwent a careful manual screening.
Study selection criteria
In this research, we rigorously incorporated research that satisfied the subsequent standards: people with cancer and treated with ICIs and evaluated the CONUT score’s value of prediction. Moreover, the aforementioned articles have documented the occurrence of at least one of the subsequent outcomes: OS, PFS, ORR, and DCR. Several other types of articles, for instance, conference abstracts, and case reports, were not included in our analysis. In cases where there is patient overlap in the research, we prioritize studies that have the most thorough data and reliable methods32.
Data extraction and quality evaluation
Two researchers manner-independently extracted the data. Any discrepancies were resolved through discussion until a consensus was reached. The collected data comprises the primary author’s name, year of publication, article design, article period, treatment, cancer type, sample capacity, age, outcome, and so on. The Newcastle–Ottawa Scale (NOS) was conducted to evaluate the quality of the individual research33. Research with NOS values surpassing 6 were categorized as studies of superior quality.
Statistical analysis
The terminal point of this meta-analysis was to predict medical outcomes for cancer patients after ICItreatment, covering OS, PFS, ORR, and DCR. The correlation between the CONUT and cancer outcome after ICI treatment was established by amalgamating the HR and the corresponding 95% CI for each included study. The heterogeneity among the studies was assessed utilizing Cochran’s Q test and Higgins I2 statistics. As in previous studies, in cases where there was notable heterogeneity (I2 > 50% or p < 0.1), the combined analysis was assessed with the random-effects model (REM) as per the DerSimonian and Laird method. Alternatively, in the absence of any notable heterogeneity, the fixed-effects model (FEM) was employed with the Inverse Variance method13. To assess the origin of heterogeneity and dependability of the findings, we performed a sensitivity analysis. Assessment of publication bias was conducted utilizing various statistical methods, including the examination of Egger’s test34 and Begg’s test35. The results were shown with funnel plots and the calculation results were also shown in the supplementary file.
Results
Literature search process and results
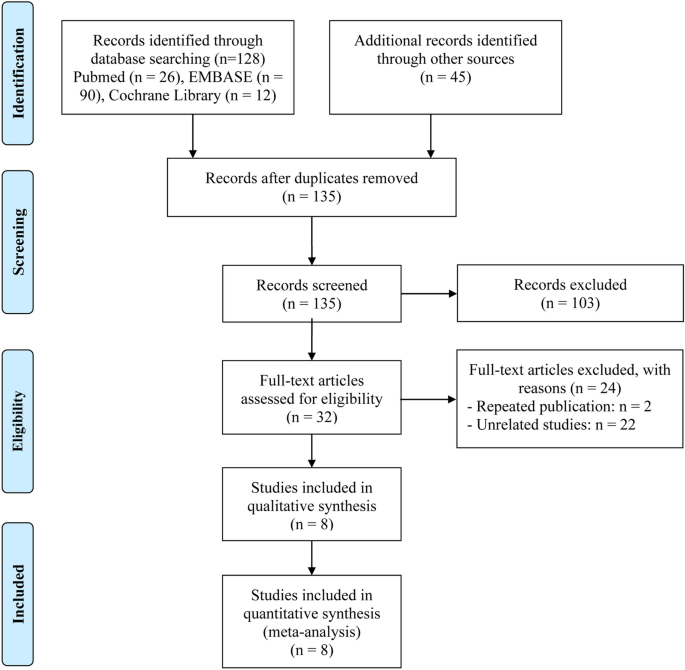
PRISMA flow diagram of the article selection process was summarized in Fig. 1. Upon conducting an initial literature search by reviewing pertinent databases, a sum of 173 articles was determined. Following the elimination of 38 duplicate articles, 135 unique articles remained for subsequent evaluation. 103 articles were deemed unsuitable for further consideration based on an evaluation of their title and abstract within the literature. Upon thorough examination, a total of 8 articles with complete text, including 663 patients were ultimately incorporated into our meta-analysis23,24, 26,27,28,29,30,31.

The flow diagram for identifying eligible studies.
Characteristics of contained articles
The salient features of the incorporated articles are succinctly outlined in Table 2. Three of these investigations were conducted in Japan23,28, 29, and five in China24,26, 27, 30, 31. The range of NOS scores observed in the research encompassed values between 6 and 9, signifying that all included studies exhibited a high level of quality. All seven studies included in the analysis were retrospective23,24, 26, 28,29,30,31, while one was single-arm study27. Two studies were conducted in patients diagnosed with ESCC patients30,31; one study was in HCC patients27, one study was in esophageal cancer (EC) patients26, one study was in GC patients24, one study was in NSCLC patients23, one study was in squamous cell carcinoma of the head and neck (HNSCC) patients28, and one study was in RCC patients29. Furthermore, 7 studies reported CONUT’s role in prognosticating OS outcome23,24, 26, 28,29,30,31, all 8 studies provided the data of CONUT for PFS prognosis23,24, 26,27,28,29,30,31, 3 studies provided the data of CONUT for DCR prognosis23,26, 28, and 4 studies provided the data of CONUT for ORR prognosis23,26, 28, 31.
Baseline CONUT levels and OS
In this study, our objective was to explore the potential correlation between CONUT levels and OS in cancer patients who were treated using ICIs. To achieve this, we conducted a comprehensive analysis of data obtained from seven independent studies, which included a total of 643 patients. The FEM was utilized as a result of the absence of significant heterogeneity (I2 = 0%, p = 0.504). As shown in Fig. 2A, the pooled HR was 1.94 (95% CI 1.52–2.47, p < 0.001) and indicated that a high CONUT score had poorer OS in cancer patients treated with immunotherapy. To assess the validity of the study's results, a sensitivity analysis was conducted. This involved examining the impact on the final results after eliminating each research from the analysis. Sensitivity analysis revealed that none of the studies significantly impacted the reported effect magnitude (Fig. 2B). When Zhao et al. was excluded, the estimated range of HR for OS changed to 1.81 (95%CI: 1.40–2.33), and when Chen et al. 2022 was excluded, the estimated range was 2.01 (95%CI: 1.61–2.74) (Fig. 2B). No publication biases in OS were observed in our study (Fig. 3, Egger’s test: p = 0.133; Begg’s test: p = 0.149).

(A) Forest plots of the association between Controlling Nutritional Status (CONUT) and overall survival (OS). HR, hazard ratio; Cl, confidence interval. (B) Sensitivity analysis of the association between CONUT and OS.

Funnel plot with pseudo 95% confidence limits. S.e. of: theta, the corresponding standard error.
Baseline CONUT levels and PFS
Furthermore, the connection between CONUT score and PFS in ICI-treated cancer patients was explored by examining the data from all 8 studies with 663 patients. Because of the significant heterogeneity, the REM was selected for further analysis (I2 = 47.8%, p = 0.063). According to Fig. 4A, the merged HR was 2.22 (95% CI 1.48–3.31, p < 0.001), indicating that a high CONUT score had worse PFS in cancer patients treated with immunotherapy. As shown in Fig. 4B, the exclusion of any specific study didn’t have an impact on the overall findings about PFS. We also observed no publication biases in PFS (Fig. 5, Egger’s test: p = 0.174; Begg’s test: p = 0.093).

(A) Forest plots of the association between CONUT and progression-free survival (PFS). HR, harzard ratio; Cl, confidence interval (B) Sensitivity analysis of the association between CONUT and PFS.

Funnel plot with pseudo 95% confidence limits in PFS.
Baseline CONUT levels and ORR
We additionally examine the association between CONUT score and ORR in cancer patients undergoing treatment with ICIs, utilizing data obtained from four studies encompassing a cohort of 251 patients. Since the heterogeneity of the included studies was not significant, a fixed-effect model was selected for further analysis (Fig. 5, I2 = 0%, p = 0.611). Based on the findings presented in Fig. 6A, the OR was 0.46 (95% CI 0.25–0.85, p = 0.013), indicating that a high CONUT score had lower ORR in cancer patients treated with immunotherapy. As Fig. 6B demonstrated, the overall result of ORR was unaffected by the removal of any one research. Moreover, publication bias was not found in the ORR (Egger’s test: p = 1; Begg’s test: p = 0.517).

(A) Forest plots of the association between CONUT and objective response rate (ORR). OR, odds ratio; Cl, confidence interval (B) Sensitivity analysis of the association between CONUT and ORR.
Baseline CONUT levels and DCR
Subsequently, we investigated the relationship between CONUT and DCR in cancer patients using data obtained from three independent studies encompassing 203 patients. Due to the heterogeneity was not significant (I2 = 0%, p = 0.577), the FEM was employed. According to Fig. 7A, the pooled HR was 0.29 (95% CI 0.14–0.59, p = 0.001). Based on the findings, it revealed that cancer patients who exhibit a high CONUT score had a decreased DCR when undergoing immunotherapy treatment. As Fig. 7B demonstrated, the overall predictive power of the DCR was unaffected by the absence of any one particular research.

(A) Forest plots of the association between CONUT and disease control rate (DCR). HR, hazard ratio; Cl, confidence interval. (B) Sensitivity analysis of the association between CONUT and DCR.
Discussion
The primary objective of our study was to investigate the prognostic value of the CONUT score in cancer patients undergoing ICI therapy. In this comprehensive meta-analysis of pertinent studies, a robust correlation has been established between a diminished CONUT score and a favorable OS and PFS, as well as an elevated ORR and DCR.
ICI has greatly increased the survival of tumor patients, but the overall clinically significant response rate is not satisfactory. Identifying patients who can distinguish between those who are likely to respond to immunotherapy will help increase patient benefit rates36. Clinical nutrition and immune-related indicator—CONUT is convenient and easy to obtain, which has been an excellent predictor in many ICI therapies. In our study, CONUT score can predict the effect of tumor immunotherapy, and tumor patients with lower CONUT scores will benefit more from immunotherapy. The prognostic implications of CONUT in cancer patients treated with ICIs can be elucidated through three fundamental components37. Specifically, albumin and total cholesterol are indicative of nutritional status, while lymphocytes serve as a reflection of immune functionality.
Clinically speaking, malnutrition frequent in cancer patients, and albumin is a widely used laboratory index in clinical practice to assess nutrition status38. At the same time, inflammation is inextricably linked to tumor progression, and there is also inflammation caused by immunotherapy. It is well known that the activation of the immune system after ICIs treatment is the major mechanism for the effectiveness of immunotherapy, and during ICI treatment, it may promote the occurrence of processes similar to inflammation39,40. Albumin is also one marker of the systemic inflammation in cancers41. The decrease in albumin levels is more pronounced in the middle and late stages of tumor patients, leading to hypoalbuminemia42,43. Hypoalbuminemia enhances the secretion of various inflammatory factors, such as interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF-α), which further stimulates tumor inflammation progression44,45. Research has demonstrated that hypoalbuminemia is a risk factor for multiple types of tumors and also contributes to elevated mortality rates associated with tumors46,47. Cholesterol, an integral constituent of cellular membranes, exhibits a strong correlation with the proliferation of tumors, and cancer patients with low cholesterol levels often have a poor prognosis48. Cholesterol also regulates the innate and adaptive immune responses of a variety of immune cells in tumors49. Cholesterol in the tumor microenvironment can reduce T cell depletion and contribute to the attenuation of the immune response against cancers50. Studies have also shown that cholesterol can enhance the anti-tumor effects of natural killer cells in mice51. This partly explains why higher CONUT scores, which correspond to lower cholesterol levels, are less beneficial for cancer patients from ICI treatment. As we know, lymphocytes are of utmost importance in modulating the immune system’s ability to combat cancer52. They effectively hinder the growth of tumor cells and exert suppressive effects on their invasive potential53. Those lymphocytes, especially tumor infiltrating lymphocytes (TILs), have been shown to actively contribute to the body’s defense against tumor progression54,55. Fewer lymphocytes, corresponding to a higher CONUT score, tend to mean a worse immune status.
In this paper, a pioneering meta-analysis that aimed to validate the prognostic utility of the CONUT in predicting the therapeutic response of cancer patients undergoing ICI therapy was accomplished. Cancer patients with low CONUT scores may potentially experience a more favorable response to ICIs. The findings of this study can help cancer patients aid in developing effective treatment strategies that facilitate the administration of precise and reduce treatment costs for cancer patients.
Admittedly, there are still limitations to our research. First of all, the predominant proportion of studies encompassed within the meta-analysis consist of retrospective studies. Secondly, owing to constraints in the number of gathered research studies, the role of CONUT in post-ICI treatment could not be explored for individual tumors. Furthermore, our analysis was limited to studies published exclusively in English and focused on data from China and Japan. This geographical restriction should be taken into consideration when interpreting the findings. Further investigation and the inclusion of more extensive sample sizes are still required in order to validate the predictive significance of CONUT in the context of ICI treatment. First, we can conduct prospective studies to validate the predictive function of the CONUT score in relation to the impact of cancer immunotherapy. Second, An increased sample size is needed to analyze a specific type of cancer and discussed the predictive capability of the CONUT score for different tumor immunotherapies (“Supplementary information”).
Conclusion
In conclusion, due to its well-established impact on the host’s nutritional and immune status as well as cancer, the CONUT score could serve as a useful tool in predicting the therapeutic outcomes of ICIs in cancer patients.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
-
Global Burden of Disease Cancer C et al. Cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life years for 29 cancer groups from 2010 to 2019: A systematic analysis for the global burden of disease study 2019. JAMA Oncol. 8(3), 420–444. https://doi.org/10.1001/jamaoncol.2021.6987 (2022).
Google Scholar
-
Sung, H. et al. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71(3), 209–249. https://doi.org/10.3322/caac.21660 (2021).
Google Scholar
-
Filin, I. Y., Solovyeva, V. V., Kitaeva, K. V., Rutland, C. S. & Rizvanov, A. A. Current trends in cancer immunotherapy. Biomedicines 8(12), 200. https://doi.org/10.3390/biomedicines8120621 (2020).
Google Scholar
-
Yadav, D. et al. Cancer immunotherapy by immune checkpoint blockade and its advanced application using bio-nanomaterials. Semin. Cancer Biol. 86(Pt 2), 909–922. https://doi.org/10.1016/j.semcancer.2022.02.016 (2022).
Google Scholar
-
Zhang, H. & Chen, J. Current status and future directions of cancer immunotherapy. J. Cancer 9(10), 1773–1781. https://doi.org/10.7150/jca.24577 (2018).
Google Scholar
-
Borghaei, H. et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N. Engl. J. Med. 373(17), 1627–1639. https://doi.org/10.1056/NEJMoa1507643 (2015).
Google Scholar
-
Motzer, R. J. et al. Nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: Extended Follow-up of efficacy and safety results from a randomised, controlled, phase 3 trial. Lancet Oncol. 20(10), 1370–1385. https://doi.org/10.1016/S1470-2045(19)30413-9 (2019).
Google Scholar
-
El-Khoueiry, A. B. et al. Nivolumab in patients with advanced hepatocellular carcinoma (Checkmate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 389(10088), 2492–5202. https://doi.org/10.1016/S0140-6736(17)31046-2 (2017).
Google Scholar
-
Weber, J. S. et al. Safety profile of nivolumab monotherapy: A pooled analysis of patients with advanced melanoma. J. Clin. Oncol. 35(7), 785–792. https://doi.org/10.1200/JCO.2015.66.1389 (2017).
Google Scholar
-
Zhang, L. et al. The correlation between probiotic use and outcomes of cancer patients treated with immune checkpoint inhibitors. Front. Pharmacol. 13, 937874. https://doi.org/10.3389/fphar.2022.937874 (2022).
Google Scholar
-
Fearon, K. C., Voss, A. C., Hustead, D. S., Cancer Cachexia Study G. Definition of cancer cachexia: Effect of weight loss, reduced food intake, and systemic inflammation on functional status and prognosis. Am. J. Clin. Nutr. 83(6), 1345–1350. https://doi.org/10.1093/ajcn/83.6.1345 (2006).
Google Scholar
-
Jiang, Y. et al. Inflammation and nutrition-based biomarkers in the prognosis of oesophageal cancer: A systematic review and meta-analysis. BMJ Open 11(9), e048324. https://doi.org/10.1136/bmjopen-2020-048324 (2021).
Google Scholar
-
Zhang, L. et al. Blood biomarkers predict outcomes in patients with hepatocellular carcinoma treated with immune checkpoint inhibitors: A Pooled analysis of 44 retrospective sudies. Int. Immunopharmacol. 118, 110019. https://doi.org/10.1016/j.intimp.2023.110019 (2023).
Google Scholar
-
Illa, P., Tomiskova, M. & Skrickova, J. Nutritional risk screening predicts tumor response in lung cancer patients. J. Am. Coll. Nutr. 34(5), 425–429. https://doi.org/10.1080/07315724.2014.938789 (2015).
Google Scholar
-
Wang, X. & Wang, Y. The prognostic nutritional index is prognostic factor of gynecological cancer: A systematic review and meta-analysis. Int. J. Surg. 67, 79–86. https://doi.org/10.1016/j.ijsu.2019.05.018 (2019).
Google Scholar
-
Ignacio de Ulibarri, J. et al. CONUT: A tool for controlling nutritional status. First validation in a hospital population. Nutr. Hosp. 20(1), 38–45 (2005).
Google Scholar
-
Liu, H. et al. Clinical significance of the controlling nutritional status (CONUT) score in gastric cancer patients: A meta-analysis of 9764 participants. Front. Nutr. 10, 1156006. https://doi.org/10.3389/fnut.2023.1156006 (2023).
Google Scholar
-
Niu, Z. & Yan, B. Prognostic and clinicopathological impacts of controlling nutritional status (CONUT) score on patients with gynecological cancer: A meta-analysis. Nutr. J. 22(1), 33. https://doi.org/10.1186/s12937-023-00863-8 (2023).
Google Scholar
-
Peng, J. et al. Prognostic value of prognostic nutritional index score and controlling nutritional status score in patients with glioblastoma: A comprehensive meta-analysis. Front. Oncol. 13, 1117764. https://doi.org/10.3389/fonc.2023.1117764 (2023).
Google Scholar
-
Takagi, K., Buettner, S. & Ijzermans, J. N. M. Prognostic significance of the controlling nutritional status (CONUT) score in patients with colorectal cancer: A systematic review and meta-analysis. Int. J. Surg. 78, 91–96. https://doi.org/10.1016/j.ijsu.2020.04.046 (2020).
Google Scholar
-
Feng, J., Wang, L., Yang, X., Chen, Q. & Cheng, X. The Usefulness of pretreatment controlling nutritional status score for predicting recurrence in patients with esophageal squamous cell carcinoma undergoing neoadjuvant immunochemotherapy: A real-world study. Front. Immunol. 13, 1015365. https://doi.org/10.3389/fimmu.2022.1015365 (2022).
Google Scholar
-
Mao, Y. S., Hao, S. J., Zou, C. F., Xie, Z. B. & Fu, D. L. Controlling nutritional status score is superior to prognostic nutritional index score in predicting survival and complications in pancreatic ductal adenocarcinoma: A Chinese propensity score matching study. Br. J. Nutr. 124(11), 1190–1197. https://doi.org/10.1017/S0007114520002299 (2020).
Google Scholar
-
Ohba, T. et al. Prognostic impact of the controlling nutritional status score in patients with non-small cell lung cancer treated with pembrolizumab. J. Thorac. Dis. 11(9), 3757–3768. https://doi.org/10.21037/jtd.2019.09.29 (2019).
Google Scholar
-
Chen, L. et al. Controlling nutritional status (CONUT) predicts survival in gastric cancer patients with immune checkpoint inhibitor (Pd-1/Pd-L1) outcomes. Front. Pharmacol. 13, 836958. https://doi.org/10.3389/fphar.2022.836958 (2022).
Google Scholar
-
Liberati, A. et al. The prisma statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 6(7), e1000100. https://doi.org/10.1371/journal.pmed.1000100 (2009).
Google Scholar
-
Chang, L. et al. Prognostic effect of the controlling nutritional status score in patients with esophageal cancer treated with immune checkpoint inhibitor. J. Immunother. 45(9), 415–422. https://doi.org/10.1097/CJI.0000000000000438 (2022).
Google Scholar
-
Chen, X. et al. Safety and efficacy of sintilimab and anlotinib as first line treatment for advanced hepatocellular carcinoma (Keep-G04): A single-arm phase 2 study. Front. Oncol. 12, 909035. https://doi.org/10.3389/fonc.2022.909035 (2022).
Google Scholar
-
Sakai, A. et al. Prognostic value of inflammatory and nutritional biomarkers of immune checkpoint inhibitor treatment for recurrent or metastatic squamous cell carcinoma of the head and neck. Cancers https://doi.org/10.3390/cancers15072021 (2023).
Google Scholar
-
Takemura, K. et al. Prognostic significance of the controlling nutritional status (CONUT) Score in patients with advanced renal cell carcinoma treated with nivolumab after failure of prior tyrosine kinase inhibitors. J. Urol. 204(6), 1166–1172. https://doi.org/10.1097/JU.0000000000001196 (2020).
Google Scholar
-
Zhang, Z. et al. Retrospective study of the combination of TP and PF regimens with or without immune checkpoint inhibitors for the first-line treatment of locally advanced or advanced esophageal squamous cell carcinoma. Ther. Adv. Med. Oncol. 15, 17588359231169980. https://doi.org/10.1177/17588359231169981 (2023).
Google Scholar
-
Zhao, X. H. et al. The prognosis value of CONUT and SIS score for recurrent or metastatic esophageal squamous cell carcinoma patients treated with second-line immunotherapy. Front. Oncol. 13, 1167625. https://doi.org/10.3389/fonc.2023.1167625 (2023).
Google Scholar
-
Zhang, L. et al. The association between antibiotic use and outcomes of HCC patients treated with immune checkpoint inhibitors. Front. Immunol. 13, 956533. https://doi.org/10.3389/fimmu.2022.956533 (2022).
Google Scholar
-
Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 25(9), 603–605. https://doi.org/10.1007/s10654-010-9491-z (2010).
Google Scholar
-
Irwig, L., Macaskill, P., Berry, G. & Glasziou, P. Bias in meta-analysis detected by a simple, graphical test. Graphical test is itself biased. BMJ 316(7129), 470 (1998).
Google Scholar
-
Begg, C. B. & Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 50(4), 1088–1101 (1994).
Google Scholar
-
Lei, Y., Li, X., Huang, Q., Zheng, X. & Liu, M. Progress and challenges of predictive biomarkers for immune checkpoint blockade. Front. Oncol. 11, 617335. https://doi.org/10.3389/fonc.2021.617335 (2021).
Google Scholar
-
Gonzalez-Madrono, A., Mancha, A., Rodriguez, F. J., Culebras, J. & de Ulibarri, J. I. Confirming the validity of the CONUT system for early detection and monitoring of clinical undernutrition: comparison with two logistic regression models developed using SGA as the gold standard. Nutr. Hosp. 27(2), 564–571. https://doi.org/10.1590/S0212-16112012000200033 (2012).
Google Scholar
-
Ravasco, P. Nutrition in cancer patients. J. Clin. Med. https://doi.org/10.3390/jcm8081211 (2019).
Google Scholar
-
Sharma, P. & Allison, J. P. The future of immune checkpoint therapy. Science 348(6230), 56–61. https://doi.org/10.1126/science.aaa8172 (2015).
Google Scholar
-
Greten, F. R. & Grivennikov, S. I. Inflammation and cancer: Triggers, mechanisms, and consequences. Immunity 51(1), 27–41. https://doi.org/10.1016/j.immuni.2019.06.025 (2019).
Google Scholar
-
Wu, N., Chen, G., Hu, H., Pang, L. & Chen, Z. Low pretherapeutic serum albumin as a risk factor for poor outcome in esophageal squamous cell carcinomas. Nutr. Cancer 67(3), 481–485. https://doi.org/10.1080/01635581.2015.1004726 (2015).
Google Scholar
-
Gupta, D. & Lis, C. G. Pretreatment serum albumin as a predictor of cancer survival: A systematic review of the epidemiological literature. Nutr. J. 9, 69. https://doi.org/10.1186/1475-2891-9-69 (2010).
Google Scholar
-
Takeda, K. et al. Impact of change in serum albumin level during and after chemoradiotherapy in patients with locally advanced esophageal cancer. Esophagus 15(3), 190–197. https://doi.org/10.1007/s10388-018-0612-1 (2018).
Google Scholar
-
Peters, S. J. et al. Co-culture of primary rat hepatocytes with rat liver epithelial cells enhances interleukin-6-induced acute-phase protein response. Cell Tissue Res. 340(3), 451–457. https://doi.org/10.1007/s00441-010-0955-y (2010).
Google Scholar
-
Honda, H. et al. Serum albumin, C-reactive protein, interleukin 6, and fetuin a as predictors of malnutrition, cardiovascular disease, and mortality in patients with ESRD. Am. J. Kidney Dis. 47(1), 139–148. https://doi.org/10.1053/j.ajkd.2005.09.014 (2006).
Google Scholar
-
Lis, C. G., Grutsch, J. F., Vashi, P. G. & Lammersfeld, C. A. Is serum albumin an independent predictor of survival in patients with breast cancer?. JPEN J. Parenter. Enteral. Nutr. 27(1), 10–15. https://doi.org/10.1177/014860710302700110 (2003).
Google Scholar
-
Cui, L. et al. Pituitary tumor transforming gene: A novel therapeutic target for glioma treatment. Acta Biochim. Biophys. Sin. 47(6), 414–421. https://doi.org/10.1093/abbs/gmv026 (2015).
Google Scholar
-
Zhou, P., Li, B., Liu, B., Chen, T. & Xiao, J. Prognostic role of serum total cholesterol and high-density lipoprotein cholesterol in cancer survivors: A systematic review and meta-analysis. Clin. Chim. Acta 477, 94–104. https://doi.org/10.1016/j.cca.2017.11.039 (2018).
Google Scholar
-
Huang, B., Song, B. L. & Xu, C. Cholesterol metabolism in cancer: Mechanisms and therapeutic opportunities. Nat. Metab. 2(2), 132–141. https://doi.org/10.1038/s42255-020-0174-0 (2020).
Google Scholar
-
Ma, X. et al. Cholesterol induces Cd8+ T cell exhaustion in the tumor microenvironment. Cell Metab. 30(1), 143–156. https://doi.org/10.1016/j.cmet.2019.04.002 (2019).
Google Scholar
-
Qin, W. H. et al. High serum levels of cholesterol increase antitumor functions of nature killer cells and reduce growth of liver tumors in mice. Gastroenterology 158(6), 1713–1727. https://doi.org/10.1053/j.gastro.2020.01.028 (2020).
Google Scholar
-
Mantovani, A., Allavena, P., Sica, A. & Balkwill, F. Cancer-related inflammation. Nature 454(7203), 436–444. https://doi.org/10.1038/nature07205 (2008).
Google Scholar
-
Kitayama, J., Yasuda, K., Kawai, K., Sunami, E. & Nagawa, H. Circulating lymphocyte is an important determinant of the effectiveness of preoperative radiotherapy in advanced rectal cancer. BMC Cancer 11, 64. https://doi.org/10.1186/1471-2407-11-64 (2011).
Google Scholar
-
Zhang, Y. & Zhang, Z. The history and advances in cancer immunotherapy: Understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol. Immunol. 17(8), 807–871. https://doi.org/10.1038/s41423-020-0488-6 (2020).
Google Scholar
-
Paijens, S. T., Vledder, A., de Bruyn, M. & Nijman, H. W. Tumor-infiltrating lymphocytes in the immunotherapy era. Cell Mol. Immunol. 18(4), 842–859. https://doi.org/10.1038/s41423-020-00565-9 (2021).
Google Scholar
Author information
Authors and Affiliations
Contributions
Z.L., Z.J., L.M., and W.W. conceived and designed the study. Z.L., L.M., Z.J., Y.J., and K.T. were responsible for the collection and assembly of data, data analysis, and interpretation. Z.J. and Z.L. were involved in writing the manuscript. Z.L., Z.J., Y.J., and W.W. revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Information.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
Reprints and Permissions
About this article
Cite this article
Zhang, J., Li, M., Zhang, L. et al. Prognostic value of controlling nutritional status on clinical and survival outcomes in cancer patients treated with immunotherapy.
Sci Rep 13, 17715 (2023). https://doi.org/10.1038/s41598-023-45096-1
-
Received: 29 August 2023
-
Accepted: 16 October 2023
-
Published: 18 October 2023
-
DOI: https://doi.org/10.1038/s41598-023-45096-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.