Abstract
Satellite technologies are essential for global conservation actions through providing continuous, real-time Earth monitoring. However, development of these technologies necessitates an increase in rocket launches, which introduces new threats to biodiversity. Here, we mapped rocket launch sites and assessed their threats on protected areas and terrestrial biodiversity. Our analysis revealed that over 90% launch sites are within areas where unprotected habitats excesses 50% and over 62% of operating sites are located within or near protected areas. The threats from rocket launches are potentially associated with biomes, coordinates, and proximity to oceans. In particular, threatened terrestrial species in Tropical and Subtropical Moist Broadleaf Forests are more vulnerable to these risks compared to species in other biomes. Without strategic planning, the continued growth of rocket launches could create conflicts between technological development and conservation efforts, undermining the achievement of UN Biodiversity Goals.
Introduction
In the Anthropocene epoch, human actions have exerted a dominant influence on the Earth system, leading to rapid environmental and climate changes, and widespread habitat destruction1,2. Consequently, anthropogenic actions have emerged as the primary driver of global biodiversity loss3,4. The decline in biodiversity driven by traditional practice of farming5, deforestation6, urbanization7, mining8, industrialization9, and transportation10 has been extensively studied. As civilizations progress and technology advances, new human activities are arising, with launching of spacecraft, rockets and satellites being one of them. These aerospace vehicles which are crucial for modern civilization, provide vital services including telecommunication, navigation, surveillance, and environmental and resource management11,12,13, expanding rapidly through ambitious launching projects. In 2023, there were 223 launch attempts, marking a 175% increase from 201314. However, threats on biodiversity and conservation efforts derived from rocket launching activities have received notably less attention compared to other human activities, which could undermine the ambitious goals of the Kunming-Montreal Global Biodiversity Framework, conserving 30% of terrestrial and marine areas by 2030 and reducing species extinction rates tenfold by 205015.
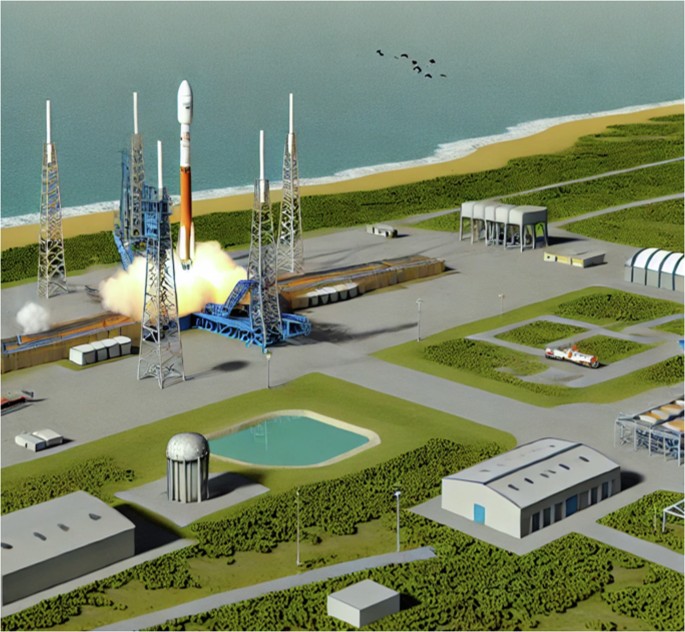
Launch activities affect earth’s surface ecosystems. During the rocket launches, explosive emissions, acoustic oscillations, and land and water use for installation drive the main ecosystem issues in the areas surrounding rocket launch sites (RLS) (Fig. 1)16,17. Shuttle cloud and exhaust of the rocket boosters can lead to local vegetation damage and biodiversity loss because of fuel spills, chemical leaks, acidic deposition and extreme noise raised during the processing18. These effects can reach up to 45 km from RLS17,19, threatening nature conservation efforts within these areas. For example, hydrochloric acid emitted from solid rocket launches leaching into nearby water led to fish kills20. Insect richness and abundance were reduced by launch events reported in up to 5 km around the Wenchang Satellite Launch Center in China21; Exposure to pollutants from launch activities, augmentation of free radical activity, DNA damage and high level of chromosomal aberrations have also been observed in little ground squirrels (Citellus pygmaeus Pallas) and house mouses (Mus musculus L.)22. Furthermore, the falling debris from separating rocket parts can extend the impact area to 400–1500 km from the launch complex of the cosmodrome23.
RLS and debris falling regions are typically selected in unpopulated areas without residents or economic activities, such as ocean and desert, where are however home for diversified and unique vegetation and wildlife, and play an important role in global ecosystem functioning24,25. For instance, a SpaceX’s launching site is the proximity of the Lower Rio Grande Valley National Wildlife Refuge in theUnited States, which contains about 1200 plants, 300 butterflies and 700 vertebrates26. Furthermore, launching accidents or failures can lead to serious ecological consequences. For example, the fire caused by Russia’s Proton Rocket Launch Crash in July 2013, covered an area of over 49,000 m2 where the soil and vegetation cover had been destroyed and many wildlife species lost their habitats27. As a consequence, more habitats will be exposured to risks associated with rocket launches considering a rapid increase in public investment and private sector in the space industry28,29.
Protected Areas (PAs) represent a fundamental strategy for conserving natural resources and ecosystems in the face of anthropogenic pressures30. Globally, over 202,000 PAs now encompass approximately 15% of the Earth’s terrestrial surface30, serving as critical refuges for threatened species31,32, especially for vertebrates33. Take Costa Rica for examples, 98.5% of threatened mammal species are represented in PAs, with all 18 endemic species, such as the mountain spiny pocket mouse (Heteromys oresterus) and Cherrie’s pocket gopher (Orthogeomys cherriei), occurring in at least three PAs34. Protected areas are often established in remote regions to minimize human impact, protect biodiversity hotspots, and preserve pristine ecosystems that face fewer conflicts over land use35. However, this preference for remote locations often leads to significant overlap with RLS. Given that PAs represent the most vital and vulnerable ecosystems on the planet, understanding how rocket launches may affect these areas is essential. Despite this growing evidence on the impacts of rocket launches on conservation, to date, no study has attempted to determine its potential risks at global scale. Here we determine the potential threats on global conservation, according to 221 pre-operational, operational, and closed RLS (Table S1). Considering that RLS are primarily located in terrestrial biomes, their environmental effects tend to be more persistent compared to those in oceanic locations, where pollution (e.g., debris) can be dispersed by ocean currents. Specifically, we address three key questions: (1) Does the selection of RLS take into account for protection status across global terrestrial biomes? (2) Is there a high proportion of RLS located within or near PAs? (3) Do rocket launches potentially impact threatened species? To address the first question, we hypothesize that if biodiversity conservation has been a factor in the selection of RLS, then biomes with higher protection status (e.g., a greater percentage of protected area) will have a less number of RLS located within or near them, reflecting the prioritization of conservation in these areas. We employed a 45 km radius around each active launch site to capture the potential influence of launches on local biodiversity conservation. To address the second question, considering there are not direct risks from launching activities in pre-operational and closed RLS, we only overlaid the locations of operating RLS with global PAs network to quantify the proportion of RLS located within and near of PAs. We then assessed the potential impact of launches on nature conservation by identifying the richness of terrestrial threatened vertebrates (i.e., amphibians, reptiles, birds and mammals) and marine hotspots near each operating RLS. Our studies provide insight on the potential impacts of RLS on PAs and global biodiversity conservation.
Results
Global RLS distribution across terrestrial biomes
Global distribution of RLS present significant spatial patterns and differences across terrestrial biomes. Generally, RLS distribution had a clear latitudinal pattern (R2 = 0.76, P < 0.01), with a strong increase in RLS number from 90°N to 30°N, up to 23% between 40°N and 30°N, then decreased to 0% between 80°S and 90°S (Fig. 2). The majority of RLS (approximately 60%) was located in the Northern Hemisphere, between 20°N and 60°N (Fig. 2). The number of RLS was positively correlated to the size of biome areas (R2 = 0.42, P = 0.013, Supplementary Fig. 1a). Temperate Broadleaf & Mixed Forests (TBMF) and Deserts & Xeric Shrublands (DXS) harbored a higher number of RLS than the remaining biomes (22.12% and 15.93%, respectively, Supplementary Fig. 2a). Montane Grasslands & Shrublands (MGS), Flooded Grasslands & Savannas (FGS) and Arctic Ice (AI) had the least RLS number (0.44%, Supplementary Fig. 2a). Rather, Mangroves (M) hosts the highest density of RLS, over five times greater than any other biome, followed by Mediterranean Forests, Woodlands & Scrub (MFWS) and TBMF (Supplementary Fig. 2a). We also found that about 67% of global RLS were situated in coasts and in oceanic areas (ocean distance), higher than these in inland areas (33%, Supplementary Fig. 2b).
a Location of required 226 launch sites including 8 pre-operation, 107 operating and 111 closed sites across 15 terrestrial biomes; b Latitudinal distribution of RLS. A Solid red line represents the output from generalized additive models and the shadow areas show the 95% confidence intervals. Detailed information for each RLS can be found in Supplementary Table 1. Data for the figure is based on all RLS including pre-operating, operating and closed sites.
RLS and global conservation priorities
RLS were located within areas designated under various protection status, predominantly Nature Could Reach Half (NCRHP, 37.61%), Nature Imperiled (NI, 26.99%), and Nature Could Recover (NCR, 26.55%), compared to a smaller proportion in Half Protected areas (HP, 8.85%; Supplementary Figs. 2c and 3). Therefore, over 90% RLS are established within areas (NCRHP, NI, and NCR) where unprotected nature habitats excess 50%. The results from linear regressions used to determine the associations between RLS and conservation category across biomes indicate that the distribution (density or number) of RLS across different biomes globally were unrelated to these protection status, PA and habitat reduction of biomes (P > 0.05; Supplementary Fig. 1). Approximately 63% of global operating RLS are within the boundaries of PA (Fig. 3 and Supplementary Fig. 4). Coastal and marine RLS have a greater conservation impact than inland sites, with over 70% overlapping with PA, versus only 42% for inland sites. Notably, only coastal and marine RLS overlap with World Heritage sites (Fig. 3). Furthermore, the marine hotspots of Central-western Pacific Ocean and Southwestern Pacific Ocean are located in the regions with higher RLS density than other marine hotspots (Supplementary Fig. 4).
RLS distribution and threatened terrestrial species
Launches in North America and East Asia exhibit central distribution patterns (Fig. 4a). Our results revealed significant relationships between RLS distribution and the richness of threatened terrestrial vertebrates, influenced by latitude (R2 = 0.467, P < 0.001, Fig. 4b and Supplementary Fig. 5) and longitude (R2 = 0.126, P < 0.001, Fig. 4c). The associations between RLS and local threatened species also vary across biomes. RLS in TSMBF within neighborhood with highest threatened species richness while lowest values to those in Tundra (T, Fig. 5a). Specifically, amphibians in TSMBF face greater risks associated with launches than those in Boreal Forests/Taiga (BFT), DXS, T, TBMF, and Temperate Grasslands, Savannas & Shrublands (TGSS) (P < 0.001, Fig. 5b). Similarly, birds and mammals in TSMBF could be more adversely impacted by launches than those in DXS, TBMF, TGSS, BFT, and T (P < 0.001, Fig. 5c, d). Reptiles also show greater susceptibility in TSMBF compared to T (P < 0.001, Fig. 5d). Additionally, biomes (R2 = 0.39, Fig. 5f) are more associated with vertebrate community composition, surpassing influences of latitude, longitude, and distinctions between inland versus coastal and marine environments (R2 = 0.03–0.13, Fig. 5f). Furthermore, we found that RLS in East and South Asia, particularly in India and southern China, are located near or within threatened biodiversity hotspots for reptiles, birds, and mammals and some RLS in East Asia (south part of China) fall within amphibian hotspots (Supplementary Fig. 5). These findings suggest that these RLS are the riskiest to threatened terrestrial species.
a Global distribution patterns of operating RLS based on our collected data; b, c their associations with local threatened species richness (log (x + 1) transformations) of total vertebrates along latitude and longitude. A Solid red line represents the output from generalized additive models and the shadow areas show the 95% confidence intervals. Data for the figure is only based on operating RLS.
a–e The differences (P < 0.05) of local richness of threatened vertebrate species (a), amphibian (b), bird (c), mammal (d) and reptile (e) threatened between biomes are shown using Kruskal–Wallis tests with Dunn’s multiple comparisons. f–h The location of RLS and their potential associations with the composition of threatened vertebrates are visualized in a graph of MNDS (f ), specially, the effects of inland vs coastal & marine (g) and biomes (h) are quantified with Kruskal–Wallis tests. Box plot showing the median (horizontal line), mean value (dot) and first and third quartiles (bottom and top of box) of the values. Data for the figure is only based on operating RLS.
Discussion
Biome conservation differences in launch threats
Differences in RLS density across biomes suggest varying conservation threats. M, which show the highest RLS density, was the most exposed biome to potential impact from launches. This could be the results that RLS were strategically situated in coasts providing main habitats for Mangroves36, to capitalize on the Earth’s eastward rotation, enhancing rocket efficiency and ensuring that rocket debris falls into the sea, thereby minimizing the risk of injury or property damage37. M is among the world’s richest storehouses of biological and genetic diversity, supporting 90% of marine organisms during part of their lifecycle and are crucial for 80% of global fish catches38. Since the damages of launch accidents and falling debris on marine biodiversity have been attended39,40, further reducing potential threats to biodiversity priority biome like M, by strengthening ecological assessments and monitoring, is crucial if conservation efforts are to secure their biodiversity.
The lack of significant associations between RLS distribution (number and density in each biome) and protection status, PA and habitat reduction indicate that biome conservation priorities were typically overlooked in the establishment of RLS. This issue probably arises from the insufficient international collaboration on assessment of potential ecological concerns posed by launch activities. Rocket launches, characterized by their extensive trajectories, are human activities whose ecological impacts are not limited to just one or two countries41,42. For instance, the use of unsymmetrical dimethylhydrazine in rocket stages, as seen with launches from Russia’s Plesetsk Cosmodrome, results in debris affecting the Arctic’s sensitive environments, including the Barents Sea and Baffin Bay43,44. This not only poses a threat to biodiversity in Russia’s areas but also raises significant ecological concerns for neighboring countries as Canada and Greenland44. Therefore, international collaborations on ecological survey, evaluation, monitor and policy decision, are the key to integrate biome-based conservation into the strategic planning of RLS establishments.
Threats of RLS to PAs
Over 60% operating RLS near or within PAs confirmed potential impacts of launches on biodiversity conservation efforts. RLS are typically located in remote areas to minimize the impact on human life and health; however, these remote areas with less disturbed natural environments often serve as habitats for diverse wildlife22,45. For example, John F. Kennedy Space Center is within Merritt Island National Wildlife Refuge, United States, which is home to 15 threatened species and provides a habitat for over 1500 species of animals and plants46,47. These RLS potentially threaten the ambitious goal set by the Kunming-Montreal Global Biodiversity Framework to conserve 30% of land, waters and seas by 2030 and reduce species extinction rates tenfold by 205015, unless effective measures are implemented to manage their ecological impacts. Despite the relatively low profile of launch activities in conservation discussions, reports of their adverse ecological impacts have emerged48,49. Space rocket accidents in Kazakhstan, for instance, have led to chemical contamination of soil and reduced vegetation cover, as well as disturbances to microbial communities, with impacts extending several kilometers from the launch pads27. Importantly, coastal and marine RLS have a greater conservation impact than inland sites, with over 70% overlapping with PAs, which suggests that coastal and marine launches require heightened attention. For example, the Brazilian space program has adversely affected coastal shark populations, with significantly elevated levels of rubidium found in apex predatory sharks near the Alcântara Space Center40. Furthermore, rocket launch debris threatens marine life within PAs: smaller fragments can cause injury or mortality through ingestion, while larger pieces disrupt seabed ecosystems by providing unnatural substrates for benthic invertebrates, altering community structures39. These potential risks are dependent on rocket technologies (e.g., types of oxidizers and fuels) and local natural factors (e.g., temperature and wind speed)48,50. Consequently, there is an urgent need to understand and quantify how these factors contribute to the risks, which is essential for informed strategic planning of launches.
If land-use conflicts between RLS and PAs are unavoidable in some countries or regions, a systematic consideration and assessment of potential risks to biodiversity is essential. Fortunately, lessons can be drawn from traditional human practices, such as mining8,51,52, to mitigate these impacts. This approach includes identifying species sensitive to disturbances associated with launches, quantifying the magnitude and footprint of these disturbances, and exploring mitigation strategies such as buffer zones and restoration techniques (e.g., soil restoration and plant rehabilitation). It is also critical to address unique risks from launches, including pollution from specific chemicals and toxicities in rocket fuels17,44. Such some study on it has started; one has shown that aluminum oxide (Al2O3) and hydrochloric acid (HCl) released from aluminized solid rocket fuel can cause neurotoxicity and metabolic depression in freshwater shrimps (Macrobrachium jelskii)53.
Potential impacts of launches on threatened species
North America and East Asia are the global centers for the distribution of operating RLS, indicating high risks from launches to threatened species within these two areas. Evidence from studies near NASA’s launch sites in Florida showed elevated levels of toxic trace elements in local wildlife, including alligators with harmful concentrations in their livers54. The use of perchlorate, a common oxidizer in rocket fuels, has been linked to thyroid disruption in amphibians and other North American wildlife, inhibiting iodine uptake and negatively affecting growth and development55. Combined with hotspot map for global threatened vertebrates, threats of launches to conservation should be highly-focused in South and East Asia (e.g., India and China). However, there are a few studies on the impact of rocket launches on conservation or biodiversity in these regions21, which is inconsistent with the rapid development of the space industry there56,57.
RLS in TSMBF within neighborhood with highest threatened species richness suggest that rocket launches in TSMBF potentially affect more threatened species than other biomes. This result could be attributed to TSMBF hosting the highest richness of threatened vertebrates compared to other biomes58. The Biodiversity Intactness Index in this biome has declined rapidly59, primarily due to intense human activities such as deforestation, urbanization, agricultural expansion, mining and infrastructure development60. Rocket launching activities, when combined with these existing pressures, could further exacerbate the vulnerability of the TSMBF biome. Therefore, the sensitivity of the TSMBF should be seriously considered and prioritized in decisions regarding launch operations and the location RLS. Our results reveal the potential impacts of launches on threatened terrestrial species and the urgent need for integrated conservation strategies that incorporate biome-specific considerations in conservation planning.
Furthermore, threatened marine species in the biodiversity hotspots of the Central-western and Southwestern Pacific Oceans are at greater risk of exposure to rocket launch activities due to the high density of RLS near these areas. These hotspots are major home to global coral reefs61, which are particularly vulnerable to launch-related disturbances. The noise from launch vehicles can damage surface coral structures62, while debris from the first and second stages of rockets may settle on the seafloor, depositing unburnt fuel, metals, and other toxic materials39. These substances can be released slowly, posing long-term risks to coral reef ecosystems. Given that marine ecosystems receive the majority of falling debris from launches, the cumulative impact of this debris could increase over time and become more significant. However, few studies have focused on the specific impacts on marine biomes or threatened species. This gap in research may be partly due to the fact that debris often falls across international boundaries, complicating cross-national studies. Future research should prioritize marine biodiversity, especially species at high risk of extinction, to better understand and quantify the impacts of rocket launches on these sensitive ecosystems.
Implications for conservation planning
The global frequency of rocket launches is expected to increase, due to the expanding demand for space and satellite technologies and reductions in launch costs63,64. Our comprehensive assessment of the potential impacts of RLS on global conservation reveals that biome type, geographic location (latitude and longitude), and whether sites are inland or coastal drive the potential impacts of launches on threatened species. This finding suggests that current launch activities do not prioritize the protection status of biomes and their associated fauna and flora. Rather, it shows that certain biomes are facing greater risks from rocket launches than others, with the highest density of RLS in M and significant risks observed in TSMBF, where many threatened terrestrial species are potentially at risk. These findings are critical for global conservation planning, as understanding the impacts of launch locations can inform decisions on global conservation priorities and future site selection. Launch pads could be strategically sited to avoid vulnerable biomes that are conservation priorities, thus mitigating potential adverse effects. This is particularly important for emerging space powers with biodiversity hotspots, such as India and China. Essential measures include assessing the ecological footprints (the influenced distances of each RLS on surrounding ecosystem functions) of launches and related infrastructure—launch pads, vehicle assembly buildings, control centers, fueling stations, and access roads—currently overlooked in international and national conservation policies. Additionally, there are currently no established ecological indicators for assessing the impact of rocket launches. Future research should focus on developing these, particularly using lichens, bryophytes, and microbiomes, which are highly sensitive to human disturbances65,66,67. Furthermore, assessing the ecological carrying capacity (ECC)68 and ecological security (ES)69 of landscapes prior to launching is crucial. Developing a quantitative-based framework for ECC and ES can guide informed decisions about RLS locations, frequency and timing of launches, fuel types, and vehicle specifications. This approach is essential for a systematic understanding of the actual, spatially explicit impacts of RLS on global conservation, beyond potential threats.
Methods
Data
Mapping global RLS
We sourced the coordinates of global RLS, including pre-operational, operational, and closed facilities, from Gunter’s Space Page (https://space.skyrocket.de/directories/launchsites.htm), Astronautix (http://www.astronautix.com), and Wikipedia (https://en.wikipedia.org/wiki/List_of_rocket_launch_sites). After verifying and confirming the accuracy of the information from each site (until May, 2023), a total of 226 RLS were selected for inclusion in this study (details in Supplementary Table 1). All required RLS were mapped using ArcGIS Pro, with a chosen radius of 45 km around each launch pad based on the findings that sonic boom pressures could extend to this distance from the launch pads and potential effects of the annoyance on biodiversity19,70. Our initial analysis aimed to include areas affected by falling debris from the second and third stages of rockets. However, the study encountered several limitations: (1) a lack of significant ecological impact reports concerning such debris, considerable variation in debris dispersal ranges among different RLS; (2) and the scarcity of publicly accessible data on debris trajectories for most launches. Consequently, we confined our research to a conservative analysis, focusing solely on the direct and indirect effects of launches based on the geographic locations of the RLS alone.
Biomes and protection status
We acquired the spatial distribution of terrestrial ecoregions and biomes from the World Wildlife Fund (www.worldwildlife.org/biomes). The initial set of 867 terrestrial ecoregions was categorized into 15 distinct biomes: (1) Arctic Ice (AI); (2) Boreal Forests/Taiga (BFT); (3) Deserts & Xeric Shrublands (DXS); (4) Flooded Grasslands & Savannas (FGS); (5) Mangroves (M); (6) Mediterranean Forests, Woodlands & Scrub (MFWS); (7) Montane Grasslands & Shrublands (MGS); (8) Temperate Broadleaf & Mixed Forests (TBMF); (9) Temperate Conifer Forests (TCF); (10) Temperate Grasslands, Savannas & Shrublands (TGSS); (11) Tropical & Subtropical Coniferous Forests (TSCF); (12) Tropical & Subtropical Dry Broadleaf Forests (TSDBF); (13) Tropical & Subtropical Grasslands, Savannas & Shrublands (TSGSS); (14) Tropical & Subtropical Moist Broadleaf Forests (TSMBF); (15) Tundra (T). The map data for protection statuses of global terrestrial biomes of the world was obtain from Dinerstein et al. 71. Four protection statuses were established to categorize terrestrial lands based on the extent of remaining natural habitat and the area under protection. These are defined as follows: ‘Half Protected (HP)’—where more than 50% of the ecoregion area is under protection; ‘Nature Could Reach Half (NCRH)’—where less than 50% of the ecoregion is protected, yet the combined total of protected areas and remaining unprotected natural habitat exceeds 50%; ‘Nature Could Recover (NCR)’—where the aggregate of PAs and remaining unprotected natural habitat falls between 20% and 50%; and ‘Nature Imperiled (NI)’—where this sum is 20% or less72. The map depicting PA, which includes terrestrial land, inland water bodies, and marine regions, was obtained from Protected Planet (https://www.protectedplanet.net). These PA encompass World Heritage sites and other designated protection categories. Data on the percentage of area protected and habitat reduction (the reduction of unprotected rare and threatened species areas in each biome) were derived from published sources73,74,75. The number of ecoregions under each protection status (Half Protected, Nature Could Reach Half, Nature Could Recover, and Nature Imperiled) in each biome was obtained from Dinerstein et al.71. Given the comprehensive and detailed assessments provided by the International Union for the Conservation of Nature (IUCN) for vertebrates, we utilized IUCN-provided distribution maps for terrestrial vertebrates76 to assess threatened species diversity. We calculated the richness of threatened species across four distinct taxonomic groups: amphibians, reptiles, birds, and mammals. The information of ecoregions, biomes, and protection statuses of each RLS within is available in Supplementary Table 1 and the threatened species richness data is in Supplementary Table 2.
Analyses
Assessing the distribution of launch sites
We overlaid maps of total RLS—encompassing closed, operating, and pre-operating facilities—with terrestrial biomes and protection statuses. This analysis was conducted to examine the associations between the spatial distribution of RLS and various biome types, as well as their impact on biodiversity conservation. To assess the relationships between the RLS number and geographic distribution, percentage of RLS within each 10-degree latitude interval and latitude were determined using generalized additive models (GAM). GAMs were fit in the R package mgcv77 using default settings. The linear regressions between conservation categories (area protected, habitat reduction, percentage of Half Protected, Nature Could Reach Half, Nature Could Recover, and Nature Imperiled) of each biome and RLS distribution (density and number, Supplementary Table 3) were calculated and the regression coefficients and the functions’ significance were obtained with the stat_poly_eq function in the ggpmisc package78.
Assessing protection effects
Considering rocket launches only occurred in operating RLS, we overlaid maps of operating RLS with PAs, to analyze differences in their proportional overlap for sites located within or near PAs versus those in non-protected areas. Marine hotspots, including the Central-Eastern Pacific Ocean, Southwestern Atlantic Ocean, Western Indian Ocean, Central-Western Pacific Ocean, Southwestern Pacific Ocean, and Oceania (central Pacific Ocean), were analyzed to examine the proximity of RLS to these biodiversity-rich areas. These six regions were identified based on global distributions of 1729 fish species, 124 marine mammal species, and 330 seabird species, highlighting their exceptional biodiversity79. Additionally, we categorized the RLS into inland and coastal/marine types to further investigate how the location of the RLS influences its proportional overlap with PAs.
Assessing threatened species effects
We overlaid maps of operating RLS (45 km offsite impacts) with data on the richness of four terrestrial taxonomic groups (amphibians, reptiles, birds, and mammals), and their total threatened species to derive richness values for each site. Only the highest richness value within each buffer zone was used to assess the risks of RLS on local threatened species. First, using the all-operating RLS data, we modeled the patterns of associated total threatened specie richness along latitude and longitude, respectively, using GAMs with a log (x + 1) transformation were used to estimate the patterns. Second, differences in local threatened terrestrial species richness between biomes were assessed using Kruskal–Wallis tests with Dunn’s multiple comparisons (datasets were not distributed normally) with the stats package80. Biomes without operating RLS and with only one site were removed. A total of 10 biomes (i.e., BFT, DXS, M, MFWS, T, TBMF, TCF, TGSS, TSGSS and TSMBF) were kept for the analysis. Third, Permutational multivariate analysis of variance (PERMANOVA) based on Bray–Curtis dissimilarity of potential associated vertebrates’ richness values, were used to determine the effects of operating launch site location on threatened species. Latitude, longitude, biomes and the location of inland vs costal & marine were included as location information in the models. Between-sample variation in threatened species composition was visualized using NMDS (non-metric multidimensional scaling). PERMANOVAs were performed using the adonis function of vegan package81.
Moran’s I was used to assess global autocorrelation of rocket launch sites buffer zone for terrestrial threatened vertebrates (total vertebrate threatened species, amphibian, bird, mammal and reptile). Values of Moran’s I less than 0 indicated negative spatial autocorrelation, as in clustering of dissimilar values, while those greater than 0 indicated positive spatial clustering, as in clustering of similar values in similar areas. The package of spde was used to calculate Moran’s I and to examine patterns of spatial autocorrelation. The Moran’s I values and P values were present in Supplementary Fig. 6. The species richness index does not account for endemism, as the goal was to provide a broad assessment of biodiversity risk around rocket launch sites. All data processing and analysis were carried out in R v.4.0.3 and a P value of <0.05 was considered statistically significant.
Data availability
The dataset collected and compiled in this study is available in the Figshare repository: https://figshare.com/s/044db09cfa88c130ab72.
Code availability
The R code used to perform the analyses is publicly available at https://github.com/RiccardiaYin/Launches-on-global-conservation.git.
References
-
Steffen, W., Grinevald, J., Crutzen, P. & McNeill, J. The Anthropocene: conceptual and historical perspectives. Philos. Trans. R. Soc. A: Math. Phys. Eng. Sci. 369, 842–867 (2011).
-
Steffen, W., Crutzen, P. J. & McNeill, J. R. THE ANTHROPOCENE: ARE HUMANS NOW OVERWHELMING THE GREAT FORCES OF NATURE? in The New World History 440–459 (University of California Press, 2019).
-
Seddon, N. et al. Biodiversity in the Anthropocene: prospects and policy. Proc. R. Soc. B: Biol. Sci. 283, 20162094 (2016).
-
Pievani, T. The sixth mass extinction: Anthropocene and the human impact on biodiversity. Rendiconti Lincei 25, 85–93 (2014).
-
Dudley, N. & Alexander, S. Agriculture and biodiversity: a review. Biodiversity 18, 45–49 (2017).
-
Liu, J. et al. Forest fragmentation in China and its effect on biodiversity. Biol. Rev. 94, 1636–1657 (2019).
-
McKinney, M. L. Effects of urbanization on species richness: A review of plants and animals. Urban Ecosyst. 11, 161–176 (2008).
-
Sonter, L. J., Ali, S. H. & Watson, J. E. M. Mining and biodiversity: key issues and research needs in conservation science. Proceedings of the Royal Society B: Biological Sciences 285, (2018).
-
Liu, Y., Xu, Y., Zhang, F., Yun, J. & Shen, Z. The impact of biofuel plantation on biodiversity: a review. Chin. Sci. Bull. 59, 4639–4651 (2014).
-
Bennett, V. J. Effects of Road Density and Pattern on the Conservation of Species and Biodiversity. Curr. Landsc. Ecol. Rep. 2, 1–11 (2017).
-
Zhao, Q. et al. An Overview of the Applications of Earth Observation Satellite Data: Impacts and Future Trends. Remote Sens (Basel) 14, 1863 (2022).
-
Kansakar, P. & Hossain, F. A review of applications of satellite earth observation data for global societal benefit and stewardship of planet earth. Space Policy 36, 46–54 (2016).
-
Hosseini, N., Jamal, H., Haque, J., Magesacher, T. & Matolak, D. W. UAV Command and Control, Navigation and Surveillance: A Review of Potential 5G and Satellite Systems. in 2019 IEEE Aerospace Conference 1–10 (IEEE, 2019).
-
Space Foundation Editorial Team. The space report 2023 Q4 shows record number of launches for third year in a row, technological firsts, and heightened focus on policy. https://www.spacefoundation.org/2024/01/23/the-space-report-2023-q4/ (2024).
-
Joly, C. A. The Kunming-Montréal global biodiversity framework. Biota Neotrop 22, (2022).
-
Carmicino, C. Acoustics, Vortex Shedding, and Low-Frequency Dynamics Interaction in an Unstable Hybrid Rocket. J. Propuls. Power 25, 1322–1335 (2009).
-
Dallas, J. A., Raval, S., Alvarez Gaitan, J. P., Saydam, S. & Dempster, A. G. The environmental impact of emissions from space launches: A comprehensive review. J. Clean. Prod. 255, 120209 (2020).
-
Schmalzer, R. et al. Environmental monitoring of Space Shuttle launches at Kennedy Space Center – The first ten years. in 31st Aerospace Sciences Meeting (American Institute of Aeronautics and Astronautics, Reston, Virigina, 1993).
-
Pearsons, K. S. An Evaluation of the Noise Impact of Satellite Power System Vehicles on the Community and Ecology at the Launch Site-Summary Report. (1980).
-
Potter, A. December. Space shuttle environmental effects: the first five flights. in Proceedings of the NASA/USAF Space Shuttle Environment Conference (Kennedy Space Center, Florida, 1982).
-
Xue, Y. et al. Rocket launching activities are associated with reduced insect species richness and abundance in two types of tropical plantations around the Wenchang Satellite Launch Center, southern China. Ecol. Indic. 127, 107751 (2021).
-
Kolumbayeva, S. et al. Chromosomal instability in rodents caused by pollution from Baikonur cosmodrome. Ecotoxicology 23, 1283–1291 (2014).
-
Koroleva, T. V. et al. The environmental impact of space transport. Transp. Res D. Transp. Environ. 58, 54–69 (2018).
-
Liquete, C. et al. Current status and future prospects for the assessment of marine and coastal ecosystem services: a systematic review. PLoS ONE 8, e67737 (2013).
-
Rocha, J. L., Godinho, R., Brito, J. C. & Nielsen, R. Life in deserts: the genetic basis of mammalian desert adaptation. Trends Ecol. Evol. 36, 637–650 (2021).
-
Faeqa, M. Optimizing Species Selection for Forest Restoration in the Lower Rio Grande Valley. (2020).
-
Koroleva, T. V., Semenkov, I. N., Sharapova, A. V., Krechetov, P. P. & Lednev, S. A. Ecological consequences of space rocket accidents in Kazakhstan between 1999 and 2018. Environ. Pollut. 268, 115711 (2021).
-
Ziemblicki, B. & Oralova, Y. Private entities in outer space activities: liability regime reconsidered. Space Policy 56, 101427 (2021).
-
Lampkin, J. & White, R. The Global Space Industry. in Space Criminology 25–47 (Springer International Publishing, Cham, 2023).
-
Le Saout, S. et al. Protected Areas and Effective Biodiversity Conservation. Science (1979) 342, 803–805 (2013).
-
Watson, J. E. M., Dudley, N., Segan, D. B. & Hockings, M. The performance and potential of protected areas. Nature 515, 67–73 (2014).
-
Hoveka, L. N., van der Bank, M. & Davies, T. J. Winners and losers in a changing climate: how will protected areas conserve red list species under climate change? Divers Distrib. 28, 782–792 (2022).
-
Quan, Q. et al. Effectiveness of protected areas for vertebrates based on taxonomic and phylogenetic diversity. Conserv. Biol. 32, 355–365 (2018).
-
González-Maya, J. F., Víquez-R, L. R., Belant, J. L. & Ceballos, G. Effectiveness of protected areas for representing species and populations of terrestrial mammals in Costa Rica. PLoS ONE 10, e0124480 (2015).
-
Joppa, L. N. & Pfaff, A. High and far: biases in the location of protected areas. PLoS ONE 4, e8273 (2009).
-
Guo, H. et al. Coastal regime shifts: rapid responses of coastal wetlands to changes in mangrove cover. Ecology 98, 762–772 (2017).
-
Rugescu, R. D. & Monea, V. Surface Corridor Requirements for Space Launches over the Black Sea. Appl. Mech. Mater. 330, 799–804 (2013).
-
Sandilyan, S. & Kathiresan, K. Mangrove conservation: a global perspective. Biodivers. Conserv 21, 3523–3542 (2012).
-
Lonsdale, J.-A. & Phillips, C. Space launches and the UK marine environment. Mar. Policy 129, 104479 (2021).
-
Wosnick, N., Chaves, A. P., Leite, R. D., Nunes, J. L. S. & Hauser-Davis, R. A. Potential negative effects of the Brazilian Space Program on coastal sharks. Front Mar Sci 10, (2023).
-
Toyoma, G. Countering Threats in Space Through International Cooperation. Space Policy 55, 101387 (2021).
-
Ross, M. N. & Jones, K. L. Implications of a growing spaceflight industry: climate change. J. Space Saf. Eng. 9, 469–477 (2022).
-
Semenkov, I. & Koroleva, T. Review on the environmental impact of emissions from space launches: a case study for areas affected by the Russian space programme. Environ. Sci. Pollut. Res. 29, 89807–89822 (2022).
-
Byers, M. & Byers, C. Toxic splash: Russian rocket stages dropped in Arctic waters raise health, environmental and legal concerns. Polar Rec. 53, 580–591 (2017).
-
Lawrence, M. J., Stemberger, H. L. J., Zolderdo, A. J., Struthers, D. P. & Cooke, S. J. The effects of modern war and military activities on biodiversity and the environment. Environ. Rev. 23, 443–460 (2015).
-
Schmalzer, P. A. & Foster, T. E. Flora and threatened and endangered plants of Canaveral National Seashore, Florida. Castanea 81, 91–127 (2016).
-
Samantha, O. An Overview of My Internship with the Ecological Program at John F. Kennedy Space Center. (2010).
-
Sharapova, A. V. et al. Snow pollution by nitrogen-containing substances as a consequence of rocket launches from the Baikonur Cosmodrome. Sci. Total Environ. 709, 136072 (2020).
-
Carlsen, L., Kenesova, O. A. & Batyrbekova, S. E. A preliminary assessment of the potential environmental and human health impact of unsymmetrical dimethylhydrazine as a result of space activities. Chemosphere 67, 1108–1116 (2007).
-
Brown, T. F. M., Bannister, M. T. & Revell, L. E. Envisioning a sustainable future for space launches: a review of current research and policy. J. R. Soc. N. Z. 54, 273–289 (2024).
-
Agboola, O. et al. A review on the impact of mining operation: monitoring, assessment and management. Results Eng. 8, 100181 (2020).
-
Gastauer, M. et al. Mine land rehabilitation: modern ecological approaches for more sustainable mining. J. Clean. Prod. 172, 1409–1422 (2018).
-
Rivera-Ingraham, G. A. et al. Are we neglecting earth while conquering space? Effects of aluminized solid rocket fuel combustion on the physiology of a tropical freshwater invertebrate. Chemosphere 268, 128820 (2021).
-
Horai, S. et al. Concentrations of trace elements in American alligators (Alligator mississippiensis) from Florida, USA. Chemosphere 108, 159–167 (2014).
-
Godfray, H. C. J. et al. A restatement of the natural science evidence base on the effects of endocrine disrupting chemicals on wildlife. Proc. R. Soc. B: Biol. Sci. 286, 20182416 (2019).
-
Tronchetti, F. & Liu, H. The 2019 notice on promoting the systematic and orderly development of commercial carrier rockets: the first step towards regulating private space activities in China. Space Policy 57, 101432 (2021).
-
Cottom, T. S. A review of indian space launch capabilities. N. Space 10, 42–50 (2022).
-
Pillay, R. et al. Tropical forests are home to over half of the world’s vertebrate species. Front Ecol. Environ. 20, 10–15 (2022).
-
De Palma, A. et al. Annual changes in the Biodiversity Intactness Index in tropical and subtropical forest biomes, 2001–2012. Sci. Rep. 11, 20249 (2021).
-
Morris, R. J. Anthropogenic impacts on tropical forest biodiversity: a network structure and ecosystem functioning perspective. Philos. Trans. R. Soc. B: Biol. Sci. 365, 3709–3718 (2010).
-
Teh, L. S. L., Teh, L. C. L. & Sumaila, U. R. A global estimate of the number of coral reef fishers. PLoS ONE 8, e65397 (2013).
-
Gee, K. L. et al. Launch Vehicle Noise and Australian Spaceports. in 040002 https://doi.org/10.1121/2.0001856 (2023).
-
Helms, C. C. A Survey of Launch Services 2016-2020. in AIAA Propulsion and Energy 2020 Forum (American Institute of Aeronautics and Astronautics, Reston, Virginia, 2020).
-
Harry, W. J. The Future Impact of Much Lower Launch Cost. (2018).
-
Abas, A. A systematic review on biomonitoring using lichen as the biological indicator: a decade of practices, progress and challenges. Ecol. Indic. 121, 107197 (2021).
-
Meyer, C. et al. Using “bryophytes and their associated testate amoeba” microsystems as indicators of atmospheric pollution. Ecol. Indic. 13, 144–151 (2012).
-
Ribas, M. P., García-Ulloa, M., Espunyes, J. & Cabezón, O. Improving the assessment of ecosystem and wildlife health: microbiome as an early indicator. Curr. Opin. Biotechnol. 81, 102923 (2023).
-
Wang, S. F. et al. Review of evaluation on ecological carrying capacity: The progress and trend of methodology. IOP Conf. Ser. Earth Environ. Sci. 113, 012108 (2018).
-
Cao, G. & Hou, P. Assessment of the ecological security based on the ecological carrying capacity. in 2016 IEEE International Geoscience and Remote Sensing Symposium (IGARSS) 7285–7288 (IEEE, 2016). https://doi.org/10.1109/IGARSS.2016.7730900.
-
Sordello, R. et al. Evidence of the impact of noise pollution on biodiversity: a systematic map. Environ. Evid. 9, 20 (2020).
-
Dinerstein, E. et al. An Ecoregion-Based Approach to Protecting Half the Terrestrial Realm. Bioscience 67, 534–545 (2017).
-
Marco Immovilli; & Marcel T.J. Kok. Narratives for the “Half Earth” and “Sharing The Planet” Scenarios: A Literature Review. (2020).
-
Dobrowski, S. Z. et al. Protected-area targets could be undermined by climate change-driven shifts in ecoregions and biomes. Commun. Earth Environ. 2, 198 (2021).
-
Vijay, V. & Armsworth, P. R. Pervasive cropland in protected areas highlight trade-offs between conservation and food security. Proc. Natl. Acad. Sci. USA 118, (2021).
-
Eric Dinerstein et al. Conservation Imperatives: Securing theLast Unprotected Terrestrial SitesHarboring Irreplaceable Biodiversity. Front. Sci. 2, (2024)
-
IUCN. Red List Version 2022-2 Species Richness. (2022).
-
Wood, S. N. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J. R. Stat. Soc. Ser. B Stat. Methodol. 73, 3–36 (2011).
-
Pedro, J. A. ggpmisc: Miscellaneous extensions to ‘ggplot2’. https://cran.r-project.org/web/packages/ggpmisc/index.html (2021).
-
Ramírez, F., Afán, I., Davis, L. S. & Chiaradia, A. Climate impacts on global hot spots of marine biodiversity. Sci. Adv. 3 (2017).
-
Kartikeya Bolar. STAT: Interactive Document for Working with Basic Statistical Analysis. version 0.1.0. https://CRAN.R-project.org/package=STAT (2019).
-
Oksanen, J. et al. Package ‘vegan’. Community Ecol. package, version 2, 1–295 (2013).
Acknowledgements
We thank Yi Ma, Zhen-Sha Luo, Xue-Kai He, Han-Di Zhou (Longquan Nature Conservation Center of Baishanzu National Park) for their help in conservation management section of the discussion. We also sincerely thank Maaike Bader and Jörg Bendix for their support in covering part of the open-access publication fee. This work was funded by the China Postdoctoral Science Foundation (Grant/Award Number: 2023M742774), the Research Project of Baishanzu National Park (2023JBGS05) and Open Fund Project of Shenzhen Key Laboratory of South Subtropical Plant Diversity. Open access funding provided by the Open Access Publishing Fund of Philipps-Universität Marburg.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
X.Y. and L.P.A. conceived and designed the project; wrote the manuscript. F.K., E.H. L.G. and Q.L. collated datasets and performed analysis with ArcGIS. F.K. performed the spatial autocorrelation analysis. X.Y. and L.L.A. performed analysis with R and generated final figures. C.C., M.J.F., L.Y., and L.S. helped with interpreting the results. All authors contributed to editing the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Earth & Environment thanks and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Alice Drinkwater. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ang, L.P., Kong, F., Hernández-Rodríguez, E. et al. Rocket launches threaten global biodiversity conservation.
Commun Earth Environ 5, 799 (2024). https://doi.org/10.1038/s43247-024-01963-x
-
Received: 21 June 2024
-
Accepted: 11 December 2024
-
Published: 31 December 2024
-
DOI: https://doi.org/10.1038/s43247-024-01963-x