Abstract
Collaborative innovation between hospitals and biomedical enterprises is crucial for ensuring breakthroughs in their development. This study explores the structural characteristics and examines the main roles of associated key actors of collaborative innovation between hospitals and biomedical enterprises in China. Using the jointly owned patent data within the country’s healthcare industry, a decade-long collaborative innovation network between hospitals and biomedical enterprises in China was established and analyzed through social network analysis. The results revealed that the overall levels of collaborative innovation network density, collaborative frequency, and network connectivity were significantly low, especially in less-developed regions. In terms of actors with higher degree centrality, hospitals accounted for the majority, whereas a biomedical enterprise in Shenzhen had the highest degree centrality. Organizations in underdeveloped and northwest regions and small players were more likely to implement collaborative innovation. In conclusion, a collaborative innovation network between hospitals and biomedical enterprises in China demonstrated high dispersion and poor development levels. Stimulating organizations’ initiatives for collaborative innovation may enhance quality and quantity of such innovation. Policy support and economic investments, strategic collaborative help, and resource and partnership optimization, especially for small players and in less-developed and northwest regions, should be encouraged to enhance collaborative innovation between hospitals and the biomedical industry in China and other similar countries or regions.
Introduction
The significance of collaborative innovation in the fields of healthcare and medicine has been well demonstrated1. Collaborative innovation is a large-span integrated organizational model, which should be carried out by multiple organizations (e.g., enterprises, governments, universities, healthcare institutions) to realize major scientific and technological innovation. Collaborative innovation is a new paradigm to break through the limitations of a single actor’s innovative capability, which has individually been unable to meet the demands for competitive advantages and economic growth2. Collaborative innovation can improve health outcomes, promote diagnosis and treatment approaches3,4,5, expand market values, maximize productivity, promote knowledge and resource sharing and exchange, and accelerate scientific and technological innovation1,6,7. In this approach, various organizations are encouraged to fully utilize their respective capabilities and advantages and integrate complementary resources under the guidance of, and according to mechanisms arranged by, the national government8. To realize such collaborative innovation and activate these advantages, the key is the strong development of various organizations (e.g., organizational vitality and capability)9. Collaborations within a single organization or between individuals have disadvantages due to limitations in capability and resource sharing and integration. Therefore, collaborative innovation between different organizations is of great significance. In China, the “Outline of the Healthy China 2030 Plan” in 2016 proposed that interdisciplinary collaboration should be the main model for the future development of medical scientific research10; collaboration between hospitals and enterprises has become an important model for innovative development11. In 2021, the “14th Five-Year Plan” for medical equipment industry development in China highlighted the principle of collaborative innovation between hospitals and enterprises and encouraged exploration of new collaborative innovation models12. Therefore, collaborative innovation between hospitals and the biomedical industry in China has been a crucial development direction.
In China, the rapid development of hospitals over recent years has resulted in high demand for innovation in clinical research, advancements in diagnosis and treatment approaches, and the transformation of medical fields. Moreover, the biomedical industry has entered a critical period of equal emphasis on research and development, transformation, and manufacturing. In 2010 and 2011, the Chinese government proposed policies that emphasized the importance of collaborative innovation between hospitals and biomedical enterprises for ensuring high-level hospital reforms and breakthroughs in the biomedical industry13,14. Therefore, since 2011, hospitals and biomedical enterprises in China have been experiencing rapid development and facing new opportunities and challenges. While the collaborative innovation between hospitals and the biomedical industry has been highlighted in China over the last ten years, little is known about whether a network system has been formed, and if so, the status of its development, successes, and failures. Additionally, more fundamental questions about such collaborations remain unclear, including identifying influential actors that accelerate collaborations and identifying potential new collaboration relationships15. This lack of clarity is closely related to the future development of both hospitals and the biomedical industry. Therefore, it is imperative to deeply explore the collaborative innovation network between hospitals and the biomedical industry.
Social network analysis (SNA) is an effective tool for analyzing and evaluating complex collaboration networks by studying the relationships and interactions between various social actors in a group16. SNA has been widely used to evaluate collaboration relationships in health care fields, which can help improve collaborations and identify influential actors who can facilitate collaborative relationships. Such SNA explorations primarily include collaborations in cancer care17, community health18,19, vaccine research20, nursing21,22, psychiatry23, medical education24, drug discovery and development25, scientific networks and organizations15,16,26,27,28,29, and scientific medical research30,31,32,33,34. These SNA studies provided evidence on collaboration features and patterns, and identified key points regarding improving collaboration levels and ranges. Therefore, SNA is a powerful tool that helps to deeply understand the collaborative innovation network between hospitals and the biomedical industry.
The collaborative innovation network reflects the teamwork referring to academic organizations, healthcare institutions, biomedical enterprises, the science community, and patients26. This highlights the vital role of the collaborative innovation relationship between hospitals and the biomedical industry. However, there is little research on the evaluation of the structure and relationships of the collaborative innovation network between hospitals and the biomedical industry using SNA. Additionally, in terms of SNA of the collaborative innovation network among other actors (except for hospitals and biomedical enterprises) mentioned above, measurements for evaluating the collaborative innovation network can be divided into two categories: publications and grants15,20,35,36 and self-reported collaborative relationships21,26,29. However, considering that the translation of research publications and grant achievements is one of the essential aims of collaborative innovation, the measurement for collaborative innovation network evaluation based on publications and grants is not appropriate. Therefore, an appropriate measure is urgently needed. Existing studies have indicated that collaborative patents is a significant form of collaborative innovation, which has a long tradition and has advantages when used to evaluate collaborative innovation37. Patents with joint ownership can accelerate the innovation process37, which further indicates the rationality of using collaborative patents as a metric by which to evaluate the development trends of collaborative innovation networks38. In addition, considering the open and available data sources of patents, their analysis to assess collaborative innovation development is feasible38. However, patents have not yet been used to evaluate the structure and relationships of the collaborative innovation network between hospitals and the biomedical industry.
Therefore, it is necessary to determine whether China’s supporting policies over the past decade have driven the formation of a collaborative innovation network and to evaluate policy effects by measuring the network through SNA and metrics obtained from jointly owned patents. This study aims to explore the structure of and relationships in the collaborative innovation network between hospitals and the biomedical industry in China over the past decade based on patents by SNA, and examines the roles of the social actors involved. This study also aims to provide a more appropriate method and perspective for elucidating such collaborations and obtaining quantitative evidence to systematically advance the systematic collaborative innovation network between hospitals and the biomedical industry in China and similar countries or regions.
Results
Quantitative characteristics of collaborative innovation
In China, the number of patents in the field of biomedicine increased gradually and steadily from 2011 to 2020. The overall trend in the number of patents jointly owned by hospitals and biomedical enterprises generally increased from 2011 to 2020, although there was a slight reduction from 2015 to 2016 and from 2018 to 2019. However, as shown in Table 1, the number of jointly owned patents accounted for a significantly small proportion of all biomedical patents (approximately 0.2%), and this proportion did not exhibit a positive trend.
Regional characteristics of collaborative innovation
The jointly owned biomedical patent network between hospitals and biomedical enterprises covered 24 regions in mainland China. The top three regions with the highest number of joint biomedical patents were China’s three most famous metropolitan areas: Beijing, Shanghai, and Guangdong (Fig. 1).

Regional distribution of different types of biomedical patents jointly owned by hospitals and biomedical enterprises in China (2011–2020).
In addition, most collaborative innovation activities of joint biomedical patent applications between hospitals and biomedical enterprises occurred in southeast China. Specifically, according to the number of joint patents, the 24 regions in this network were divided into five levels: 77–229, 38–76, 15–37, 6–15, and 1–5. The largest number (the first level) includes the three regions of Beijing, Shanghai, and Guangdong, indicating the strongest collaborative innovation development. The second level includes the two regions of Sichuan and Jiangsu, suggesting good collaborative innovation development. The third to fifth levels include five, seven, and seven regions, respectively, revealing weak collaborative innovation development status (Fig. 2). Furthermore, to examine whether the number of collaborative innovation activities is correlated with geographical locations of organizations, a quadratic assignment procedure (QAP) was conducted. The QAP result indicated a positive correlation among organizations in northwest China, and a negative correlation among organizations in southeast China and between organizations in northwest and southeast China. This result indicates that collaborative innovation is less likely between organizations in northwest and southeast China and among organizations in southeast China, while organizations in northwest China are more likely to practice collaborative innovation (Table 4).

Regional distribution of biomedical patents jointly owned by hospitals and biomedical enterprises in China (2011–2020).
Furthermore, the most biomedical patents jointly owned by hospitals and biomedical enterprises are distributed in the more economically developed regions. Specifically, according to values of regional gross domestic product (GDP) in 202039, the 24 regions in this network were divided into five ranks:≥ 10, 5–10, 3–5, 1–3, and < 1 trillion CNY. Although there are only 13 regions for ranks 1 to 3, 91.4% of biomedical patents jointly applied and owned by hospitals and biomedical enterprises are in these economically developed regions. Regions that have not yet experienced collaborative innovation activities of joint patent applications (without joint patents) are all in economically undeveloped areas (regions in ranks 4 and 5; Table 2). Additionally, the QAP examined whether the number of collaborative innovation activities is correlated with regional economic development levels. The QAP result suggests a positive correlation among organizations in economically undeveloped areas, a negative correlation between organizations in economically developed and undeveloped areas, and no correlation among organizations in economically developed areas. This result indicates that collaborative innovation is less likely between organizations in economically developed and undeveloped areas, while organizations in economically undeveloped areas are more likely to practice collaborative innovation (Table 4).
Professional field characteristics of collaborative innovation
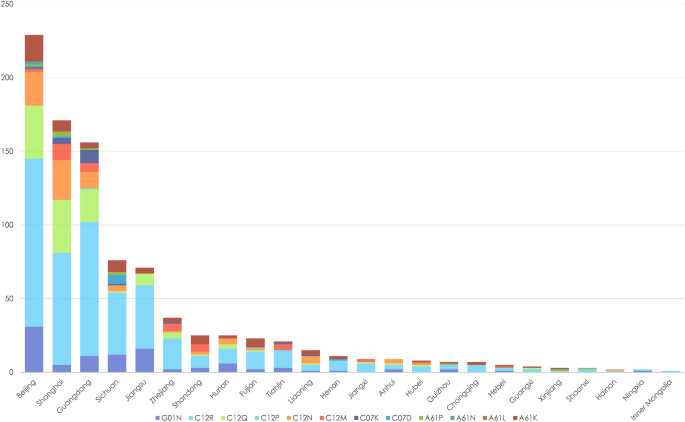
The top five classifications of biomedical patents jointly owned by hospitals and biomedical enterprises in China were G01N, C12N, A61K, A61P, and C12Q, according to IPC numbers. Patent applications for C12M, C07K, C12R, C12P, and C07D were significantly fewer (Fig. 3).

Professional distribution of jointly owned biomedical patents in China from 2011 to 2020.
Overall network analysis
The collaborative innovation network between hospitals and biomedical enterprises was established based on the overall number of jointly owned patents from 2011 to 2020; the trend over time was not considered. Therefore, this is an undirected one-mode network. It has 341 nodes, which include 144 hospitals, 197 biomedical enterprises, and 460 edges between these nodes, representing joint patent partnership (Fig. 4). The overall network density is 0.004, thereby indicating that the network relationship is at a low concentration level, the overall network structure is sparse, the connectivity is relatively weak, and the degree of resource sharing is not high. Additionally, the collaborative innovation network is not a connected graph. This undirected network has 113 components, and there is a giant component with 70 nodes (Supplementary file). Most nodes at the edge of the network only have one connection, and the overall network is still in the low-frequency collaborative stage.

Visual diagram of the collaborative innovation network between hospitals and the biomedical industry.
Centrality analysis
Degree centrality indicates the most active institutions in the collaborative innovation network. The analysis result shows that the top two institutions with the highest degree centrality are Shenzhen Huada Gene Technology Co., Ltd., and the Beijing Union Medical College Hospital of the Chinese Academy of Medical Sciences, with 11 and 10 partners, respectively (Table 3). The distribution characteristic of degree centrality conforms to the exponential distribution (Kolmogorov–Smirnov test, P < 0.001).
In addition, the top 25 institutions with the highest degree centrality are clustered in seven regions, of which six regions are economically developed areas (Table 2). Specifically, most are located in Shanghai (8/25), Beijing (7/25), and Guangdong (6/25); Sichuan, Hunan, Liaoning (economically undeveloped region), and Jiangsu each have one institution (Table 3).
Furthermore, actors are considered big players if the degree centrality is at least 3.000; otherwise, they are conceived as small players. Table 3 shows all 25 big players, of which 80% (20/25) are hospitals, while only 20% are biomedical enterprises (5/25). Moreover, QAP was conducted to examine whether the number of collaborative innovation activities are correlated with the type of players. The QAP result revealed a negative correlation among big players, a positive correlation among small players, and no correlation between big and small players. This result indicates that collaborative innovation is less likely between big players, while small players are more likely to practice collaborative innovation (Table 4).
Discussion
The collaborative innovation network from 2011 to 2020 based on jointly owned biomedical patents in mainland China included 144 hospitals and 197 biomedical enterprises, and involved 460 connections. Although the number of biomedical patents jointly owned by hospitals and biomedical enterprises showed an increasing trend, the total amount and its proportion of all biomedical patents were still small, especially in less-developed regions; collaborative innovation is more likely between organizations in less-developed regions while collaborative innovation between developed and less-developed regions is less likely. This jointly owned biomedical patent network comprised 24 regions in mainland China and presented obvious regional characteristics. Most biomedical patents jointly owned by hospitals and biomedical enterprises were primarily distributed in southeast China, especially in China’s three most famous metropolitan areas: Beijing, Shanghai, and Guangdong; collaborative innovation is more likely between organizations in northwest China, while collaborative innovation between organizations in southeast China or between organizations in southeast and northwest China is less likely. Patents related to the G01N, C12N, and A61K classifications were the most popular for ensuring collaborative innovation. The overall network is an undirected one-mode network and not a connected graph. This network is sparse with a density of 0.004, and has 113 components with a giant component including 70 nodes. A biomedical enterprise in Guangdong and a hospital in Beijing have the highest degree centrality, and actors with higher degree centrality are mainly hospitals and clustered across six economically developed regions. There are 25 big players in the network; however, collaborative innovation is less likely between these big players and collaborative innovation between small players is more likely. Overall, after experiencing ten-year collaborative innovation accelerating policy efforts in China, from the perspective of joint patents, the collaborative innovation network between hospitals and biomedical enterprises is still very sparse and without good connectivity.
This study used collaborative patent statistics to observe and assess collaborative innovation networks between hospitals and biomedical industry for the first time, which clearly indicated China’s policy effects over the past decade. The usage of collaborative patents provided a feasible and reasonable method and perspective by which to quantitatively elucidate collaborative innovation networks in the biomedical field. The characteristics, structure, relationships, and roles of innovation entities can be comprehensively analyzed. Moreover, the collaborative innovation network established in this study provides the following evidence by which to strengthen the existing network and develop new collaborations.
Although the level of collaborative innovation between hospitals and the biomedical industry in China remains low, the attention paid to collaborative innovation keeps increasing. The overall quantitative tendency in joint patents continues to increase. Nonetheless, jointly owned patents were far fewer than the total in the field of biomedicine, for the following reasons. First, in China, there is a large gap in collaborative innovation between hospitals and the biomedical industry between various regions with different levels of economic development. Our findings are consistent with existing evidence that the number of patents might be correlated with the level of regional economic development40. Our results show that the number of collaborative patents is fewer in both the less-developed regions and the northwest China, and the two findings are essentially consistent; that is, the number of patents jointly owned by hospitals and biomedical enterprises is much lower in the economically less-developed regions of China. However, it is important to note that although the current number is low, hospitals and biomedical enterprises in economically underdeveloped regions and northwest China are more motivated to implement collaborative innovation. Therefore, investments or policy support should be guaranteed in these regions to create a better environment for collaborative innovation between hospitals and the biomedical industry22. Second, research statistics suggest that the transformation rate of hospitals, even in developed regions of China, such as Shanghai, is only 3%, which is considerably lower than the international transformation rate of approximately 30–50%41. Furthermore, low transformation rates are associated with low densities and frequencies of collaborative innovation networks between hospitals and the biomedical industry41. Third, evidence shows that in China, only 5% of primary drugs are available among all approved biological drugs42. Investments from biomedical enterprises aiming to fund innovative research are significantly low, and this attribute hinders the actualization of collaborative innovation between hospitals and the biomedical industry. However, such investments must satisfy the requirements associated with both the scale and capital strength of biomedical enterprises. According to a study conducted in the United States, the median research and development investment for a new drug was $985.3 million, and the mean investment was $1.336 billion43. Moreover, over recent years, the levels of investment required to ensure innovative research from both public and private sources keep increasing44, thereby suggesting the potential for creating collaborative innovation networks between hospitals and biomedical enterprises.
According to IPC analysis, the most jointly owned biomedical patents in China are those involving the G01N (investigating or analyzing materials by determining their chemical or physical properties), C12N (microorganisms or enzymes; compositions thereof; propagating, preserving, or maintaining microorganisms; mutation or genetic engineering; culture media), A61K (preparations for medical, dental, or toilet purposes), A61P (specific therapeutic activity of chemical compounds or medicinal preparations), and C12Q (measuring or testing processes involving enzymes, nucleic acids or microorganisms; compositions or test papers thereof; processes of preparing such compositions; condition-responsive control in microbiological or enzymological processes) classifications. These types of patents are generally in accordance with popular trends reported in other studies45. Identifying such trends can not only suggest the future professional direction of collaborative innovation in China46, but guide policy-makers to prioritize resources and partnerships to strengthen the existing collaborative innovation network and promote new collaborations20.
Considering that most of the actors with higher degree centrality are hospitals, more hospitals are active in practicing collaborative innovation activities than the biomedical industry. This aspect may be related to the gap in the development stages of hospitals and biomedical enterprises. Owing to the concepts and practices employed in research hospitals47, hospitals in China have experienced rapid development over the past two decades48, and this has been a national medium and long-term development plan49. Since the main task of research hospitals is conducting innovative research and collaborative translation50,51, hospitals have stronger initiatives for seeking and actualizing collaborative innovation52. However, similar practices and policy directions were not proposed in the field of biomedicine until a decade ago14. This attribute explains the delayed development in the collaborative innovation of biomedical enterprises compared with hospitals. Furthermore, the whole network is still sparse and without good connectivity, thereby indicating that the collaborative innovation capabilities of both hospitals and biomedical enterprises are very weak and far from facilitating the development of a collaborative innovation network for breaking through the current elementary stage53. It is worth noting that small players are more likely to practice collaborative innovation, suggesting a direction to increase the connectivity of the network by identifying potential small players and providing more policy support and initiatives for them.
There are several limitations associated with this study. First, considering that the scope of the IPC in the field of biomedicine varies across different studies, the biomedical patents included in this study are based on a broader range, which can be made highly accurate in future studies. Second, patent data were obtained from only IncoPat. The sources of patent data should be increased in future studies. Third, we selected the first two applicants if there were three or more applicants, thereby neglecting some actors who made small contributions to the collaborative innovation network and some new relationships. Fourth, although it is reasonable to evaluate the levels of collaborative innovation using the metric of patents jointly owned by hospitals and biomedical enterprises, a comprehensive measurement metric including aspects other than patents can be used to better reflect collaborative innovation levels in future studies. Fifth, although the aim of this study was to examine whether the ten-year efforts on collaborative innovation of China’s supporting policies have driven the network formation and analyze its in-depth characteristics, studying the network’s evolution over time could be interesting and valuable. However, such changes have not been considered currently, which should be included in future studies.
Conclusions
This study describes and analyzes a decade-long collaborative innovation network structure and relationships between hospitals and the biomedical industry based on jointly owned patents. Policy support and increased investment strategy should be encouraged to improve collaborative innovation density and frequency, especially in less-developed and northwest regions. The government should further enhance the quality and quantity of collaborative innovation by stimulating the collaborative innovation initiatives of hospitals and inspiring the potential of developing more biomedical enterprises. To break through the dispersed and low connectivity limitation, it is necessary to strengthen the existing collaborative innovation network, and promote new collaborations. Policy-makers should strategically help small players establish more partnerships and big players improve collaborative innovation capabilities.
Methods
Study design
Owing to rapid advancements in the fields of medicine and biomedicine in China over the past decade, the collaborative innovation network between hospitals and the biomedical industry in China was constructed based on patents jointly owned by hospitals and biomedical enterprises from 2011 to 2020. The adjacency matrix of patent applicants was constructed using Excel 16.16.27. The generated matrix was imported into UCINET 6.732 (Harvard: Analytic Technologies) to generate the network topology of the collaborative innovation network and analyze the network’s cohesion and centrality metrics54.
Data source
The patent data jointly owned by hospitals and biomedical enterprises were collected from the IncoPat scientific and technological innovation intelligence platform (BEIJING INCOPAT CO., LTD.). Patents with biomedical-related codes were extracted according to the International Patent Classification (IPC) system. The extraction process was also based on keywords obtained from existing studies to ensure comprehensiveness.
The inclusion criteria involved the following aspects: (1) patents were jointly applied and authorized by at least one hospital and one biomedical enterprise; (2) the application was in mainland China; (3) the patent type was a valid patent for invention; (4) the application time was from January 1, 2011 to December 31, 2020; (5) IPC codes were A61P (special therapeutic activity of chemical compounds or medicinal preparations), A61K (preparations for medical, dental, or toilet purposes), C07K (peptides), C07H (sugars; derivatives thereof; nucleosides; nucleotides; nucleic acids), C12N (microorganisms or enzymes; compositions thereof; propagating, preserving, or maintaining microorganisms; mutation or genetic engineering; culture media), C12M (apparatus for enzymology or microbiology), C12P (fermentation or enzyme-using processes to synthesize a desired chemical compound or composition or separate optical isomers from a racemic mixture), C12Q (measuring or testing processes involving enzymes, nucleic acids or microorganisms; compositions or test papers thereof; processes for preparing such compositions; condition-responsive control in microbiological or enzymological processes), and G01N (investigating or analyzing materials by determining their chemical or physical properties); (6) keywords were searched, including cell, gene, interferon, interleukin, hormone, recombination, protein, enzyme, antibody, monoclonal antibody, antigen, receptor, fermentation, nucleic acid, amino acids, nucleotides, peptides, serum, coagulants, diagnostic reagents, inhibitors, cross-linking agents, vaccines, vitamins, traditional Chinese medicine, and equipment; (7) the first two applicants were included if there were three or more applicants for a patent; and (8) patent application regions included the regions of the first two applicants. The exclusion criteria were: (1) repeatedly applied patents; (2) patents not related to the field of biomedicine; (3) patents applied by organizations and individuals from other countries; (4) patents owned by individuals; and (5) patents owned only by hospitals or biomedical enterprises.
Network visualization and metrics
The analysis of the collaborative innovation network includes visualization and quantitative metrics. In terms of visualization, the actor in the network is usually represented as a circle or square, and its size in this network reflects the number of patents. The lines between two actors represent the collaborative innovation between them.
Quantitative metrics can be divided into metrics at the network and individual levels. Metrics at the network level include the number of nodes, the number of edges, density, and components. The number of nodes refers to the number of all patent-owning institutions included in the network. The number of edges refers to the number of connections between nodes, which is the total number of cooperative relationships between patent owners. Network density is an index for evaluating the internal information connectivity of the network, which reveals the closeness between the collaborative innovation network and the collaborative relationship of biomedicine in China55. Component analysis can be used to test the connectivity of the network and identify the giant component.
In terms of metrics at the individual level, centrality measures are the most common in SNA. Because this network is not a connected graph, only degree centrality holds significance. Degree centrality is used to measure the degree of communication between a node and other nodes in a network. If a node has a high number of links, it is in a highly important and central position in a network56. QAP is used to explore the correlation between attribute features and the collaborative innovation relationship.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
-
Hyrkas, P. et al. Collaborative innovation in healthcare: A case study of hospitals as innovation platforms. Int. J. Value Chain Manag. 11, 24–41. https://doi.org/10.1504/ijvcm.2020.105475 (2020).
Google Scholar
-
Haken, H. Synergetics: An Introduction (Springer, 1977).
Google Scholar
-
Gordon, A. C. & Russell, J. A. Innovation and safety in critical care: Should we collaborate with the industry? Pro. Intensive Care Med. 44, 2276–2278. https://doi.org/10.1007/s00134-018-5306-6 (2018).
Google Scholar
-
Hanson, J. D. et al. Using social network analysis to visualize networks in American Indian Health Research. Collaborations (Coral Gables, Fla.) https://doi.org/10.33596/coll.22 (2019).
Google Scholar
-
Raad, R. & Appelbaum, P. S. Relationships between medicine and industry: Approaches to the problem of conflicts of interest. Annu. Rev. Med. 63, 465–477. https://doi.org/10.1146/annurev-med-061410-121850 (2012).
Google Scholar
-
Brittin, J. et al. From competition to collaboration: How a multi-firm research coalition is realizing rigorous facility evaluation at Parkland Hospital. HERD 13, 32–45. https://doi.org/10.1177/1937586719879717 (2020).
Google Scholar
-
Wadmann, S. Physician-industry collaboration: Conflicts of interest and the imputation of motive. Soc. Stud. Sci. 44, 531–554. https://doi.org/10.1177/0306312714525678 (2014).
Google Scholar
-
Chen, J. & Yang, Y. J. Theoretical basis and connotation of collaborative innovation. Sci. Res. 30, 161–164. https://doi.org/10.16192/j.cnki.1003-2053.2012.02.001 (2012).
Google Scholar
-
Lu, J. J. Construction of innovative carriers in the collaborative development of industry, academia, research and innovation from the perspective of organizational sociology: With a discussion on the future development of industry education consortia. Commun. Vocat. Educ. 29–39 (2023).
-
Committee, C. P. o. C. C. Outline of the Healthy China 2030 Plan (2016).
-
Ma, L. & Gao, S. S. Exploration of the construction of clinical research laboratories for medical enterprise integration. China Health Resour. 26, 711–714. https://doi.org/10.13688/j.cnki.chr.2023.230329 (2023).
Google Scholar
-
Ministry of Industry and Information Technology, N. H. C., National Development and Reform Commission & Department of Science and Technology Ministry of Finance, S. A. f. M. R., National Medical Security Administration, State Administration of Traditional Chinese Medicine, National Medical Products Administration. “14th Five-Year Plan” for medical equipment industry development, 2021).
-
Zhang, W. Ministry of science and technology takes four measures to promote the development of the biomedicine industry. Chin. Med. Biotechnol. 5, 320 (2010).
-
Ministry of Science and Technology, M. o. H., Food and Drug Administration, Chinese Medicine Bureau, Ministry of Education, National Population and Family Planning Commission, Chinese Academy of Sciences, Chinese Academy of Engineering, Natural Science Foundation, General Logistics Department and Ministry of Health. Notice on Issuing the “12th Five-Year Plan” for Medical Science and Technology Development. Ministry of Science and Technology of the People’s Republic of China: Beijing (2011) (2011).
-
Bian, J. et al. Social network analysis of biomedical research collaboration networks in a CTSA institution. J. Biomed. Inform. 52, 130–140. https://doi.org/10.1016/j.jbi.2014.01.015 (2014).
Google Scholar
-
Long, J. C., Hibbert, P. & Braithwaite, J. Structuring successful collaboration: A longitudinal social network analysis of a translational research network. Implement Sci. 11, 19. https://doi.org/10.1186/s13012-016-0381-y (2016).
Google Scholar
-
Steitz, B. D. & Levy, M. A. A social network analysis of cancer provider collaboration. AMIA Annu. Symp. Proc. 2016, 1987–1996 (2016).
Google Scholar
-
White, K. B., Resmondo, Z. N., Jennings, J. C., Creel, L. M. & Kelly Pryor, B. N. A social network analysis of interorganisational collaboration: Efforts to improve social connectedness. Health Soc. Care Community 30, e6067–e6079. https://doi.org/10.1111/hsc.14044 (2022).
Google Scholar
-
Simpson, V. L., Hass, Z. J., Panchal, J. & McGowan, B. Understanding the development, evaluation, and sustainability of community health networks using social network analysis: A scoping review. Am. J. Health Promot. 36, 318–327. https://doi.org/10.1177/08901171211045984 (2022).
Google Scholar
-
Chen, J. et al. Evolution of global scientific collaboration in mRNA vaccine research: Insights from bibliometric and social network analysis (2010–2023). Hum. Vaccin. Immunother. 19, 2276624. https://doi.org/10.1080/21645515.2023.2276624 (2023).
Google Scholar
-
Cato, K. D. et al. Nurse and midwife researcher collaboration in eastern sub-Saharan Africa: A social network analysis. Int. Nurs. Rev. 66, 571–576. https://doi.org/10.1111/inr.12542 (2019).
Google Scholar
-
Hou, X. N., Hao, Y. F., Cao, J., She, Y. C. & Duan, H. M. Scientific collaboration in Chinese nursing research: A social network analysis study. Comput. Inform. Nurs. 34, 47–54. https://doi.org/10.1097/cin.0000000000000181 (2016).
Google Scholar
-
Wu, Y. & Duan, Z. Social network analysis of international scientific collaboration on psychiatry research. Int. J. Ment. Health Syst. 9, 2. https://doi.org/10.1186/1752-4458-9-2 (2015).
Google Scholar
-
Santen, S. A. et al. Social network analysis of publication collaboration of accelerating change in MedEd consortium. Med. Teach. 44, 276–286. https://doi.org/10.1080/0142159x.2021.1985096 (2022).
Google Scholar
-
Cheng, F., Ma, Y., Uzzi, B. & Loscalzo, J. Importance of scientific collaboration in contemporary drug discovery and development: A detailed network analysis. BMC Biol. 18, 138. https://doi.org/10.1186/s12915-020-00868-3 (2020).
Google Scholar
-
Ho, E. et al. Fostering interdisciplinary collaboration: A longitudinal social network analysis of the NIH mHealth Training Institutes. J. Clin. Transl. Sci. 5, e191. https://doi.org/10.1017/cts.2021.859 (2021).
Google Scholar
-
Bailie, J., Matous, P., Bailie, R. & Passey, M. E. Patterns of collaboration and knowledge generated by an Australian rural research centre over 20 years: A co-authorship network analysis. Health Res. Policy Syst. 21, 87. https://doi.org/10.1186/s12961-023-01029-y (2023).
Google Scholar
-
Petrescu-Prahova, M. et al. Using social network analysis to assess mentorship and collaboration in a public health network. Prev. Chronic Dis. 12, E130. https://doi.org/10.5888/pcd12.150103 (2015).
Google Scholar
-
Hoe, C. et al. Using social network analysis to plan, promote and monitor intersectoral collaboration for health in rural India. PLoS ONE 14, e0219786. https://doi.org/10.1371/journal.pone.0219786 (2019).
Google Scholar
-
Hashemi-Madani, N., Emami, Z. & Khamseh, M. E. International scientific collaboration on pituitary research: A social network analysis. Med. J. Islam. Repub. Iran 35, 194. https://doi.org/10.47176/mjiri.35.194 (2021).
Google Scholar
-
Guo, D. et al. Mapping the scientific research on integrated care: A bibliometric and social network analysis. Front. Psychol. 14, 1095616. https://doi.org/10.3389/fpsyg.2023.1095616 (2023).
Google Scholar
-
Duffett, M., Brouwers, M., Meade, M. O., Xu, G. M. & Cook, D. J. Research collaboration in pediatric critical care randomized controlled trials: A social network analysis of coauthorship. Pediatr. Crit. Care Med. 21, 12–20. https://doi.org/10.1097/pcc.0000000000002120 (2020).
Google Scholar
-
Yu, S. Y. & Wang, H. M. Scientific collaboration: A social network analysis based on literature of animal-derived regenerative implantable medical devices. Regen. Biomater. 3, 197–203. https://doi.org/10.1093/rb/rbw019 (2016).
Google Scholar
-
Qin, Y. & Chen, R. Social network analysis of COVID-19 research and the changing international collaboration structure. J. Shanghai Jiaotong Univ. Sci. https://doi.org/10.1007/s12204-022-2558-7 (2022).
Google Scholar
-
Santen, S. A. et al. Social network analysis of publication collaboration of accelerating change in MedEd consortium. Med. Teach. https://doi.org/10.1080/0142159x.2021.1985096 (2021).
Google Scholar
-
Nagarajan, R. et al. Social network analysis to assess the impact of the CTSA on biomedical research grant collaboration. Clin. Transl. Sci. 8, 150–154. https://doi.org/10.1111/cts.12247 (2015).
Google Scholar
-
Liu, W., Song, Y. & Bi, K. Exploring the patent collaboration network of China’s wind energy industry: A study based on patent data from CNIPA. Renew. Sustain. Energy Rev. 144, 110989. https://doi.org/10.1016/j.rser.2021.110989 (2021).
Google Scholar
-
Liu, W., Tao, Y., Yang, Z. & Bi, K. Exploring and visualizing the patent collaboration network: A case study of smart grid field in China. Sustainability 11, 465 (2019).
Google Scholar
-
National Bureau of Statistics. (ed National Bureau of Statistics) (2020).
-
Duan, X., Wang, J., Wang, Y. W. & Lin, L. Z. Analysis of the correlation between patent and regional economic level in China. Value Eng. 38, 178–182. https://doi.org/10.14018/j.cnki.cn13-1085/n.2019.15.053 (2019).
Google Scholar
-
Kang, Q. & Du, X. L. Research on promoting the docking development of Shanghai’s medical and health service industry and biomedical industry. Sci. Dev. https://doi.org/10.3969/j.issn.1674-6171.2021.03.001 (2021).
Google Scholar
-
Wang, X. F. Preliminary exploration on the development situation and countermeasures of Guangdong Biomedical Industry. Guangdong Econ. 36–41 (2021).
-
Wouters, O. J., McKee, M. & Luyten, J. Estimated research and development investment needed to bring a new medicine to market, 2009–2018. Jama 323, 844–853. https://doi.org/10.1001/jama.2020.1166 (2020).
Google Scholar
-
Qiu, L., Chen, Z. Y., Lu, D. Y., Hu, H. & Wang, Y. T. Public funding and private investment for R&D: A survey in China’s pharmaceutical industry. Health Res. Policy Syst. 12, 27. https://doi.org/10.1186/1478-4505-12-27 (2014).
Google Scholar
-
Xue, Q. et al. Analysis and research on patent layout of biomedicine industry. China Invent. Patent 19, 19–26 (2022).
-
Xia, T. S., Wang, Y. L. & Tian, L. L. Research on present situation and countermeasure of Jiangsu biomedical business based on patent. China Biotechnol. 36, 123–130. https://doi.org/10.13523/j.cb.20160816 (2016).
Google Scholar
-
Strangeways, T. S. The work of the Cambridge Research Hospital. Br. Med. J. 2, 1529–1531 (1913).
Google Scholar
-
Jiang, C. B. et al. Building a research-oriented hospital through science and education. Chin. J. Med. Sci. Res. Manag. 62–64 (2003).
-
Zhao, L. et al. Control costs, enhance quality, and increase revenue in three top general public hospitals in Beijing, China. PLoS ONE 8, e72166 (2013).
Google Scholar
-
Li, X. X., Liu, H. F., Hao, Y. W. & Zheng, J. C. The history and development direction of research hospital. Chin. Hosp. 20, 20–22 (2016).
-
Wang, F. Q., Chen, F. & Chen, J. H. Scientific connotation of the research-oriented hospital development strategy. Hosp. Adm. J. Chin. People’s Lib. Army 19, 7–9. https://doi.org/10.16770/j.cnki.1008-9985.2012.01.011 (2012).
Google Scholar
-
Nachev, P., Herron, D., McNally, N., Rees, G. & Williams, B. Redefining the research hospital. NPJ Digit. Med. 2, 119. https://doi.org/10.1038/s41746-019-0201-2 (2019).
Google Scholar
-
Gao, Y. S., Shi, T. & Tang, J. The current situation and prospect of biomedical R&D infrastructure in Shanghai. Shanghai Med. Pharm. J. 38, 51–54 (2017).
-
Borgatti, S. P., Everett, M. G. & Freeman, L. C. UCINET for Windows: Software for Social Network Analysis (Analytic Technologies, 2002).
-
Khalagi, K. et al. Co-authorship network analysis of Iranian researchers on osteoporosis. Arch. Osteoporos. 16, 74. https://doi.org/10.1007/s11657-021-00914-9 (2021).
Google Scholar
-
Golbeck, J. Chapter 3: Network structure and measures. In Analyzing the Social Web (2013).
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (72104140, 82101870, 72274209), Soft Science Project of Shanghai Science and Technology Innovation Action Plan (23692115000, 23692113200), Shanghai Pujiang Program (21PJC083), Zhejiang Provincial Natural Science Foundation of China (LQ21H100001), Shanghai Municipal Health Commission Program (20234Y0070), and Hangzhou Health Science and Technology Program (B20220431).
Author information
Authors and Affiliations
Contributions
X.L. and W.Y. conceived the project. Y.L., J.Z., and J.T. collected the data. H.C., and M.L. performed the network analyses. Y.L. and T.T. performed all the numerical calculations. X.L. wrote the manuscript. All authors reviewed and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Table S1.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
Reprints and permissions
About this article
Cite this article
Liu, X., Chen, H., Liu, Y. et al. Social network analysis of a decade-long collaborative innovation network between hospitals and the biomedical industry in China.
Sci Rep 14, 11374 (2024). https://doi.org/10.1038/s41598-024-62082-3
-
Received: 17 January 2024
-
Accepted: 13 May 2024
-
Published: 18 May 2024
-
DOI: https://doi.org/10.1038/s41598-024-62082-3
Keywords
- Social network analysis
- Collaborative innovation
- Network
- Hospital
- Industry
- Biomedicine
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.