Abstract
A true circular carbon economy must upgrade waste greenhouse gases. C1-based biomanufacturing is an attractive solution, in which one carbon (C1) molecules (e.g. CO2, formate, methanol, etc.) are converted by microbial cell factories into value-added goods (i.e. food, feed, and chemicals). To render C1-based biomanufacturing cost-competitive, we must adapt microbial metabolism to perform chemical conversions at high rates and yields. To this end, the biotechnology community has undertaken two (seemingly opposing) paths: optimizing natural C1-trophic microorganisms versus engineering synthetic C1-assimilation de novo in model microorganisms. Here, we pose how these approaches can instead create synergies for strengthening the competitiveness of C1-based biomanufacturing as a whole.
Introduction
The development of a circular, bio-based economy is one of the grand challenges of this century. In fact, the use of biology for manufacturing (henceforth referred to as biomanufacturing) can be instrumental towards mitigating greenhouse-gas emissions into the atmosphere while supporting economic growth1,2,3. Biomanufacturing is historically rooted in bioprocesses using polytrophic organisms2,4, meaning heterotrophic organisms that grow on multicarbon feedstocks5. CO2 is fixed by crops into biomass, which is treated to release plant sugars for their biological conversion into value-added products4 by microbial cell factories6.
Although plants can successfully fix CO2 at ambient concentrations, they display a low energy conversion efficiency. It has been estimated that only 4.6–6% of sunlight energy is captured in biomass in C3 and C4 plants, respectively7,8. Moreover, empirical measurements of such conversions report that efficiencies can get lower than 3%9,10. Another drawback of plant-based production is the broader negative impact on planetary boundaries, including nitrogen- and phosphate-cycles, land-system change, fresh water use and the use of xenobiotics for pest control11. Efficient use of lignocellulosic residues is complicated by recalcitrance, which imposes the use of further inputs in energy and materials to extract plant sugars12,13. In addition, easily accessible sugars (e.g. starch, sucrose) directly compete with the food supply chain. Altogether, these characteristics render biomanufacturing fueled by plant-biomass uncompelling for a circular carbon economy capable of mitigating greenhouse gases emissions while limiting species loss, land and water use, and the introduction of xenobiotics in the ecosystems.
The use of photosynthetic microorganisms (e.g. cyanobacteria or microalgae) for biomanufacturing is also being explored extensively14,15,16. This is justified by several factors: being unicellular organisms, their cellular biomass is photosynthetically active; they present a faster growth-rate; do not require scarce resources such as arable land or freshwater; can be harvested throughout the whole year16. Therefore, they could be used as cell factories for the synthesis of value-added compounds. However, the reported values of photosynthetic efficiency for these species in pilot-scale reactors are in the order of 1.8–4.2%17,18. This, combined with major challenges in scaling up photosynthetic cultivations (i.e. contamination of open systems, low light availability along the optical path which require extensive land use) render the industrial application of photosynthetic microorganisms inadequate for large-scale biomanufacturing19,20,21.
An alternative approach can rely on the use of photovoltaic solar cell modules to capture solar energy with reported conversion efficiencies for this technology reaching values of about 24%22,23. Electricity is then used to generate biologically available electron donors that can be used in microbial fermentations. The hydrogen evolution reaction can generate hydrogen (H2) from water with high selectivity and H2 can be used as an energy source in fermentations with CO2 as the carbon source. Alternatively, CO2 itself can be electrochemically upgraded into other more reduced C1-feedstocks3,24,25 (Fig. 1). These molecules (e.g. carbon monoxide, formate, methanol, and methane) can be generated with different efficiencies25,26,27,28,29 and can serve as both carbon and energy source. A variety of different microorganisms can then assimilate these C1 feedstocks (Fig. 2). In this article, we will refer to organisms able to grow on one-carbon compounds as C1-trophic organisms, as previously defined30.

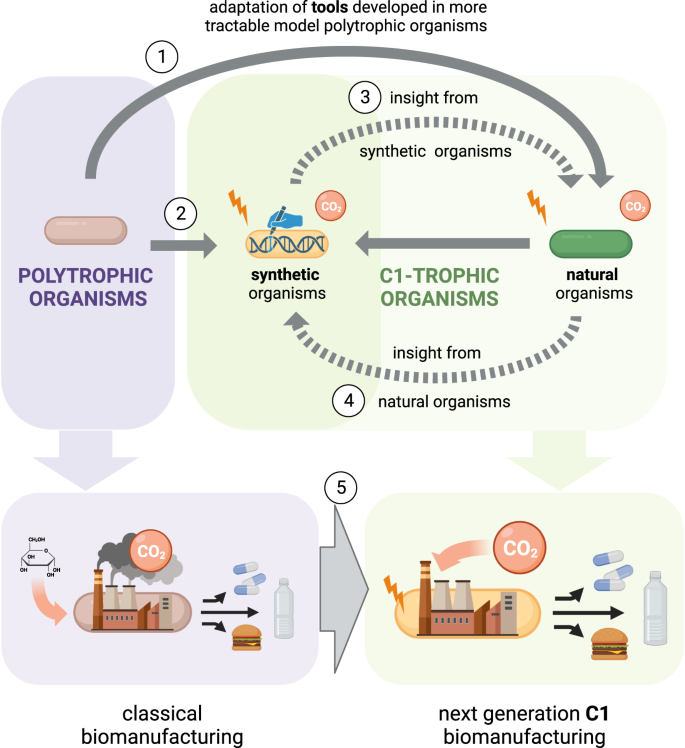
Systems and synthetic biology tools are usually developed in model polytrophic organisms, and are then applied to non-model organisms (1). Recently, some polytrophic organisms have been engineered to grow on C1 carbon sources (2). We argue that insight generated from such synthetic systems can help to improve natural C1-trophic organisms (3), which in turn should be studied to guide further engineering approaches (4). A next-generation of cell factories engineered to assimilate C1 feedstock could enable the switch from classical biomanufacturing to C1-based biomanufacturing (5). Created using BioRender.com.

C1 lifestyles are ordered by the level of reduction of the C1 compounds assimilated. At the intersection between polytrophic organisms and C1-trophic organisms lie the recently constructed synthetic C1-trophic microorganisms. Created using BioRender.com.
Different levels of technological readiness (TRL) exist for each feedstock, with the highest values of TRL 9 reported for the electrochemical synthesis of H2 and a TRL 7–9 for the microbial conversion of C1-feedstocks like methanol, methane, and syngas28. These approaches are already superior to natural photosynthesis in terms of solar-to-product energy conversion31,32,33,34. Consequently, a plethora of studies exists in literature that describe the conversion of C1-feedstocks to value-added compounds via microbial cell factories35,36,37, with the first bioprocesses recently reaching industrial scale38.
Currently, most of the cell factories used for C1-based biomanufacturing are non-model microorganisms (Fig. 2). Their physiology is less understood than polytrophs such as e.g., Escherichia coli and Saccharomyces cerevisiae. Moreover, such non-traditional species present a less developed genetic toolkit than model polytrophs39,40,41,42. Because of these limitations of non-model species, a complementary approach has emerged in the past decade, which consists of rewiring the core metabolism of polytrophs to accommodate a C1-trophic behavior by means of synthetic biology. Illustrative examples will be discussed in a separate section in more detail and include, e.g., the implementation of the Calvin-Benson-Bassham (CBB) cycle43, the reductive glycine pathway (rGlyP)44,45, the ribulose monophosphate (RuMP) cycle46,47, and the serine threonine cycle (STC)48 in the model bacterium E. coli.
Current domains of synthetic biology and metabolic engineering for C1-feedstocks seemingly follow two distinct routes. On one hand, natural C1-trophic organisms are being studied and engineered to become more standardized and superior cell factories49,50,51,52,53,54; on the other hand, polytrophs which present well characterized metabolism and for which we possess advanced genome editing toolkits are being rewired to grow on C1-feedstocks by hefty synthetic biology endeavors36. This distinction has been highlighted in a recent, timely opinion article which contrasts natural with synthetic trophic modes (in the context of methanol assimilation), highlighting the inferior growth rates and product yields of synthetic methylotrophs55.
Although the two approaches seem to diverge or even compete against each other, we believe that they can contribute synergistically to the advancement of C1-based biomanufacturing (Fig. 1). In this perspective article, we aim to highlight the possible connections existing between the investigation of natural and synthetic organisms that are capable of using C1-feedstocks for growth. By using the schematic presented in Fig. 1, we define the links that already exist, and that we propose, between model and non-model organisms as well as natural and synthetic C1-trophic organisms. Each of these links are addressed in a dedicated section. Altogether, we reason that the fields of systems and synthetic biology can support this overarching goal for pushing forward C1-based biomanufacturing. We will also discuss how these links can advance our comprehension of C1 metabolism and its further optimization for a circular carbon economy.
Systems and synthetic biology tools from model polytrophic to non-traditional C1-trophic organisms
The historical development of systems and synthetic biology tools enabled a transition from methods exclusively available for model organisms to their expansion to non-canonical microbial species (Fig. 3). The advent of omics technologies in the 1990s and early 2000s enabled the systematic investigation of living systems as a whole, leading to the emergence of systems biology as a standalone discipline56. This has been facilitated by using model organisms such as E. coli and S. cerevisiae. In fact, as these species are abundant human commensals57, they have been used as models in biology since the first half of the 20th century58. Therefore, they represented ideal platforms around which to develop system biology tools, as our understanding of their genetics and physiology was already quite advanced.

Created using BioRender.com. Source data are provided as a Source Data file.
The field of genetic engineering is considered to have started in the 1970s with the first recombinant DNA technologies. In particular, this decade was pivotal for reaching important milestones in molecular biology, such as the use of restriction enzymes, DNA recombination, and DNA sequencing59. The genome editing revolution brought on by CRISPR-based genetic engineering in the mid 2010s60 significantly accelerated the synthetic biology revolution. This breakthrough was also initiated in the two abovementioned model species, with first landmark works on the topic published in the early 2010s61,62. From that moment onwards, scientists have been able to investigate and engineer biological systems in an unprecedented way, expanding the use of CRISPR-Cas tools also to non-model species39,40,63,64 (Fig. 3).
Both E. coli and S. cerevisiae rely on a polytrophic lifestyle. Therefore, exploiting those organisms for method development has clear advantages, as it relies on energetic carbon feedstocks, which enable fast growth and hence increase the speed of technological development. Moreover, since the dawn of molecular biology, many genetic elements (e.g. plasmids, origin of replications, promoters, ribosome binding sites, etc.) have been characterized for these species. All this caused the establishment and validation of most new protocols and genetic engineering approaches using these organisms. Conversely, newly isolated microorganisms pose a challenge in terms of genetic manipulation and have much less characterized genetic parts. For this reason, the adaptation of novel systems and synthetic biology technologies has been lagging in non-model microorganisms, including C1-trophs. For example, only in the last few years toolboxes for genetic engineering were adapted for non-model C1-trophic organisms (Fig. 3). Nevertheless, although genome-editing tools of C1 assimilating microorganisms are still in their infancy, we can expect a significant increment in the amount of species domesticated through these tools in the years to come40.
Synthetic C1-trophic metabolism
The technological advances highlighted in Fig. 3 have enabled the engineering of synthetic metabolisms at different scales65. Synthetic metabolism has been defined as the subfield of synthetic biology that attempts to engineer metabolic routes that do not exist in nature66. Here, we refer to synthetic metabolism as those synthetic biology and metabolic engineering efforts that rewire the metabolic network in an organism, often by introducing heterologous metabolic pathways or modules. In recent years, many efforts focused on engineering synthetic C1-trophy (e.g. autotrophy, formatotrophy or methylotrophy, Fig. 2), mimicking natural metabolic pathways that can assimilate C1, extensively reviewed elsewhere36.
In the case of the CBB cycle, two articles served as steppingstones towards the implementation of full synthetic autotrophy. The first milestone was reached in 2016 with the generation of a hemi-autotrophic E. coli strain synthesizing all its sugar phosphates through the CBB cycle67. Then, in 2018 the same cycle was functionally implemented in Methylobacterium extorquens, although it did not succeed in supporting full autotrophic growth68. The first complete demonstration of transforming a polytrophic organism into an autotroph was published in 2019, when E. coli was equipped with the CBB cycle to grow on CO2 and formate as sole carbon and energy sources43. In the same year, the methylotrophic yeast Pichia pastoris (now called Komagataella pastoris) was also converted into an autotrophic organism capable of growth on CO2 and methanol using the CBB cycle69. Apart from engineering synthetic autotrophy, in several studies non-methylotrophic heterotrophic organisms were transformed to attain a methylotrophic metabolic lifestyle. The reductive glycine pathway (rGlyP), first theorized as a synthetic pathway70 and then discovered to be natural71, was successfully implemented into E. coli44,45, in the knallgas bacterium Cupriavidus necator72, and in the soil bacterium Pseudomonas putida for growth on formate73,74. Anticipating the generation of a fully functional rGlyP, stepwise approaches with the validation of modular segments of the route were described for E. coli and S. cerevisiae75,76,77. Moreover, the rGlyP has been shown to also support methanol assimilation45,74. Similarly, the ribulose monophosphate (RuMP) cycle was implemented in E. coli for growth on methanol46,47. Also in the case of the RuMP cycle, several preliminary works, which did not realize a complete autocatalytic cycle, achieved important milestones towards that goal78,79,80,81. Altogether, these works provided valuable insight regarding the plasticity of microbial metabolism and demonstrated our ability to control and rewire metabolic networks.
Another approach to engineering synthetic metabolisms, is to design and implement completely synthetic pathways. Rather than relying on the metabolic repertoire present in nature70, this approach enables the creation of much more efficient routes to assimilate C1. A handful of new-to-nature synthetic CO2 fixation pathways have been created and tested in vitro in the last year. The first of such works included the crotonyl-CoA/ethylmalonyl-CoA/hydroxybutyryl-CoA (CETCH) cycle82. Then, the reductive Glyxoxylate and Pyruvate Synthesis (rGPS) cycle and the Malyl-CoA-Glycerate (MCG) pathway were combined into the rGPS-MCG cycle, which was shown to reach CO2 rates up to 0.55 mM/h83. Recently, the HydrOxyPropionyl-CoA/Acrylyl-CoA (HOPAC) cycle was also functionally assembled in vitro, converting about 3.0 mM of CO2 within two hours84. A synthetic variant of the serine cycle, the serine-threonine cycle, was first proposed by Arren Bar-Even85 and then implemented by his group in E. coli, demonstrating a new synthetic formate assimilation route in vivo48.
As a result of these engineering campaigns, our overall understanding of synthetic C1 assimilation is improving. The mutations responsible for autotrophy in E. coli were recently identified, and a strain with such (surprisingly few) mutations grew with comparable growth rates86. In the synthetic methylotrophic E. coli, only the expression of three heterologous genes and deletion of two genes were required to induce methylotrophy47 albeit at low growth rates, which could be improved after an adaptive laboratory evolution experiment of 250 generations.
Despite these successful attempts, the engineering of synthetic C1-trophic strains is still in its infancy. All strains constructed so far have lower growth rates and yields than their natural counterparts. For example, the evolved methylotrophic E. coli strain has a doubling time of 8 h47, which is still far from the 3 h doubling time of the natural methylotroph Methylobacillus flagellatus87. While C. necator can naturally grow on formate (through formate oxidation and the CBB cycle) with a doubling time of 3 h88, the fastest synthetic formatotrophic strain to date has a doubling time of 6 h89. Hence, there is significant room for improvement before synthetic C1 assimilating strains can be exploited for C1-based biomanufacturing.
Understanding C1 metabolism through synthetic metabolism approaches
In the previous section, we presented the achievements in terms of synthetic C1-trophic routes within model microorganisms. Thanks to their better-characterized metabolism and genetic accessibility, polytrophs are ideal platforms in which to understand metabolic design principles that can be applied to the optimization of one-carbon metabolisms. In this section, we convey the idea that knowledge obtained from synthetic metabolism endeavors can facilitate our understanding, and consequent optimization, of metabolism and physiology in natural C1-trophic organisms. We provide four complementary examples supporting this thesis focused on investigating design principles of autocatalytic cycles, identifying possible evolutionary trajectories of carbon concentrating mechanisms, surpassing biomass yield in the natural CBB cycle via the rGlyP, and enhancing the thermodynamic drive of bioproduction pathways.
Most of the C1-assimilation pathways described in literature rely on autocatalytic cycles, namely pathways where one of the products is a catalyst for the pathway itself. The CBB cycle is an example of autocatalytic route because for every five molecules of ribulose 1,5-BP it consumes, it generates six ribulose 1,5-BP while fixing five CO2 molecules. To better understand the autocatalytic nature of the CBB cycle, a fundamental study of the dynamics and stability of autocatalytic cycles corroborated the importance of changes in substrate affinity of enzymes at the cycle’s branch points90. This evidence was confirmed in a further investigation, where the genetic basis of E. coli’s adaptation to a functional CBB cycle -described in the previous section43,67– was assessed in further detail through retro-engineering91. Mutation in three genes (prs, serA, and pgi) prevented efflux from the cycle towards biomass precursors. Moreover, modifications at two global regulators (crp and ppsR) proved to be essential for the transition from polytrophy to autotrophy. In a follow-up study looking at different lineages of evolved autotrophic strains, only 3 mutations (pgi, crp and rpoB) were identified and proved to be essential to enable autotrophy in E. coli86. The mutations were involved in preventing efflux from the CBB cycle (pgi) and increasing the redox potential (crp, rpoB). Introducing these mutations in a wild-type E. coli strain enabled autotrophy at similar rates to strains that were evolved for several generation86. By exploiting the naïve metabolic context of a polytrophic host, these findings provided mechanistic understanding for the implementation of a robust, autocatalytic CO2 fixation cycle.
In a similar fashion, E. coli was used as platform in which to reconstruct a bacterial CO2 concentrating mechanism (CCM)92. This study empirically demonstrated that ca. 20 genes from Halothiobacillus neapolitanus are required for successful CO2 fixation through Rubisco at ambient air condition. Together, these genes encode for an α-carboxysome structure capable of containing Rubisco and carbonic anhydrase as well as a transporter for inorganic carbon. E. coli harboring the α-carboxysomes from H. neapolitanus was further employed to determine the stoichiometry and the plasticity of such microcompartment93. The same carboxysome has been further employed in E. coli together with the expression of Rubisco activase components to enhance CO2 fixation94. Moreover, the E. coli strain harboring the complete CCM was used as control platform in which to study the paths of CCM evolution in autotrophic bacteria95. From a biotechnological perspective, a whole bacterial CCM has been heterologously expressed in autotrophic eukaryotes to enhance carbon fixation, e.g., by introducing synthetic α-carboxysomes within tobacco chloroplasts96.
The rGlyP is particularly remarkable as it is highly efficient in energetic terms, and it runs aerobically70,97. Even though the pathway was eventually discovered in nature, most studies around this route have focused on introducing it into polytrophic microorganisms. However, due to its high efficiency, its exploitation could be of interest also to improve growth in natural C1-trophic organisms. In fact, this was recently achieved by successfully implanting the pathway in C. necator72. Eventually, after further rounds of engineering and evolution, it has been postulated that such a synthetic strain could grow with higher yields (~15%) on formate compared to the wild type growing with the CBB cycle98. This evidence could be very impactful, suggesting that in the future relying on efficient synthetic C1 assimilation pathways might be of interest even in organisms naturally capable of growth on C1.
Principles of synthetic metabolism developed in polytrophs can also benefit bioproduction from C1 feedstocks. Of particular interest are those options that expand the design space for pathway optimization99. One example of this sort is represented by the malonyl-CoA bypass, which originates from Streptomyces sp. and was first realized in E. coli for the synthesis of mevalonate100. This bypass allows synthesis of acetoacetyl-CoA via the condensation of acetyl-CoA with malonyl-CoA (through combined activity of acetyl-CoA carboxylase and NphT7) instead of the condensation of two acetyl-CoA molecules (through acetoacetyl-CoA thiolase). Whereas the latter route is thermodynamically unfavorable (ΔrG’m > 0 kJ/mol), the malonyl-CoA bypass results in a favorable reaction thanks to the investment of one ATP ((ΔrG’m < 0 kJ/mol). In terms of bioproduction from C1 feedstocks, this bypass has been harnessed for enhancing photoautotrophic 1-butanol synthesis in S. elongatus101 and formate-dependent production of crotonate in C. necator88.
In summary, the studies presented in this section demonstrate how insight obtained in synthetic strains could be used to understand and improve C1 assimilation even in natural strains for biotechnological applications.
Principles of natural C1-trophic organisms to guide the engineering of synthetic organisms
While studying synthetic organisms is a novel approach that can provide understanding into natural phenomena, insight from natural C1-trophic organisms has been (and still is) important for the engineering of synthetic metabolisms. Such organisms play a significant role in the planetary carbon cycle, and they have fine-tuned their metabolism over billions of years of evolution. We argue that modern quantitative systems biology studies of such natural non-model organisms can provide detailed blueprints to engineer synthetic strains. While many genome sequences were made available in the last decades and polytrophic microorganisms have been investigated thoroughly at the systems-scale, such studies of natural C1-trophic organisms are only starting to be performed.
For example, functional genomics studies have become fundamental to determine the function and essentiality of genes at a high throughput. Transposon insertion sequencing is a common method to verify the genotype–phenotype relationship of knockout mutants, which has been applied extensively in the last decade102. However, only in past few years this approach was applied to C1-trophic organism, more specifically on C. necator103,104 and C. autoethanogenum105. An alternative approach is by using pooled libraries of CRISPRi strains, in which the genotype-phenotype relationship can be investigated through knocking down gene expression over time106,107,108. In 2022, Eubacterium limosum was subjected to such a study, revealing the organism’s gene fitness essentiality in autotrophic and heterotrophic growth conditions109.
On the other hand, proteomics and metabolomics are powerful methodologies that can help us obtain quantitative understanding of how a cell is built and are particularly important for the investigation of metabolism. For example, it was shown that cells need to invest many resources in the expression of carbon assimilation enzymes. Accordingly, acetogens are known to devolve up to one third of their proteome to C1-trophic functions110. This high fraction of the proteome is not controlled tightly at the expression level, while fluxes are controlled at the thermodynamic level, by sensing changes to energy levels in the metabolome111,112. Similarly, the methanogen M. maripaludis utilizes up to one fourth of its proteome for methanogenesis113. Remarkably, the proteome maintains a constant allocation upon changes in growth rates, unlike for polytrophic microorganisms in which the anabolic and catabolic fractions of the proteome are regulated based on nutrient availability114. Also the facultative knallgas bacterium C. necator retains high expression levels of C1-trophic enzymes, even when grown on a multi-carbon source like fructose103,115. In fact, analyzing the metabolome and metabolic fluxes of C. necator also suggested that when grown on multi-carbon sources, the organism maintains a mixotrophic metabolic regime116,117, which appears to be an investment in readiness to quickly restore autotrophic growth103. Hence, it appears that natural microorganisms have evolved to maintain their proteome at high capacity for growth on C1 substrates, regardless of their presence in the environment. Understanding which other genes are regulated in different conditions might pinpoint unknown mechanisms by which natural strains maintain homeostasis. To this end, methodologies such as independent component analysis of transcriptome data might help reconstructing in detail the gene regulatory network, but have not been applied so far to natural C1-trophic organisms118. Combining reconstructed gene regulatory networks with quantitative metabolomics methods of C1 metabolism119,120 in different conditions could be used to pinpoint which metabolites are involved in mechanisms that change gene expression121 and in maintaining metabolic homeostasis122.
Given the high proteome fraction needed for C1-trophic growth, furthering our understanding of the regulation of enzymatic catalytic rates is of key importance. However, this is still a challenge even in microorganisms like E. coli, in which metabolite-protein interactions are widely unknown123. Novel systematic methodologies might help in the quest to unravel these interaction networks also in natural C1 assimilating strains124,125. For example, a recent study employed one of such systematic methods (LiP-SMap, limited proteolysis-small molecule mapping) proposing novel allosteric interactions for enzymes of the CBB cycle of photoautotrophs and of C. necator126. Another recent work shed light on the allosteric inhibition of the phosphoketolase XPK of Synechococcus elongatus PCC7942, revealing how the enzyme responds to low ATP levels to drain CBB cycle’s intermediates towards the formation of acetyl-phosphate127.
In summary, insight into how nature has shaped C1-trophic organisms can streamline the engineering of synthetic organisms, providing basic design principles. In fact, synthetic evolved strains often end up mimicking natural metabolic architectures. For example, when evolving synthetic methylotrophy in E. coli47, levels of the methanol dehydrogenase increased significantly (up to 40% of the proteome), and fluxes rearranged so that TCA anaplerosis would conserve carbon, through the PEP carboxylase or malic enzyme, similarly to what can be observed in natural methylotrophs128. Guiding engineering campaigns with insight into the architecture of natural C1 metabolism and its regulation can therefore help in speeding up the construction of robust synthetic strains.
An outlook on next generation C1-based biomanufacturing
Although natural organisms have evolved to grow optimally on C1 in the environment, they are not immediately suitable for C1-based biomanufacturing. Such organisms might need to be adapted or engineered to become fermenterphiles, meaning microorganisms perfectly adapted to grow in the environment of a bioreactor129. In fact, there have been few successful cases of C1-based biomanufacturing at the industrial scale to date, which all relied on using natural C1-trophic strains, either adapted/evolved or genetically engineered. Natural methanotrophs are exploited to produce single-cell protein for animal feed (Calysta, calysta.com; Unibio, unibio.dk). More recently, engineered acetogenic bacteria were used to convert C1 compounds into C2 and C3 compounds in a carbon-negative fashion (Lanzatech)38.
On the other hand, the utilization of synthetic C1-trophic organisms is in its early days, as synthetic strains are often unfit for efficient growth and bioproduction. Despite some initial proof-of-concepts89,130, to the best of our knowledge, there are no cases of synthetic C1-trophic organisms used for large-scale biotechnological processes. Achieving an effective plug&play implantation of C1 assimilation in established cell factories will open the possibility of quickly testing it into different genetic backgrounds. This might be particularly important as different species might be innately more or less tolerant to high concentrations of C1 compounds73,88,131. As many polytrophic microorganisms have been dominating the biotech industry in the past, a wealth of information is available about their physiology and upscaling to industrial settings.
When feeding cells with C1 feedstocks, preventing resource waste (i.e. excessive oxidation of the substrate) will also become of key importance to render C1-based biomanufacturing economically competitive. Preventing resource waste will be fundamental to adopt C1-based biomanufacturing in life support systems used for space exploration applications, in which resources are limited and need to be recycled constantly132,133,134. In this context, the utilization of more efficient synthetic assimilation pathways should lead to higher yields. Evolving or engineering enzymes with higher catalytic rates might enable the construction of strains with a lower protein burden in catabolic enzymes, capable of more efficient growth135. Moreover, the utilization of minimal cell factories can play a big role in preventing resources diverting into useless portions of biomass. Model heterotrophic microorganisms have been widely engineered for this purpose136. On the other hand, genome reduction of natural C1 assimilating microorganisms is still lagging, with a single very promising recent achievement: in a recent study, it was demonstrated that a genome reduced strain of C. necator grows with higher growth rates and slightly higher yields137. Combining genome reduction approaches with synthetic assimilation pathways will eventually support efficient biomass or product formation from C1 feedstocks.
In the near future, it is likely that industrial processes will continue to rely on unmodified or engineered natural strains with purely synthetic strains quickly catching up. The choice of the initial organism will be influenced by regulatory frameworks, especially in places where rules for genetically engineered organisms are stricter (e.g. the European Union). In either case, combining the knowledge obtained in natural and synthetic strains will speed up the process of constructing more efficient strains and deliver a next generation of C1-trophic biofactories. Eventually, the adoption of such engineered strains will have to be considered along other challenges at the level of bioprocess engineering129 towards ultimately supporting efficient C1-based biomanufacturing.
Throughout this perspective article we propose a call to action for the communities working on natural- and synthetic-C1 trophic organisms to join their efforts in studying C1 metabolism as a whole. We believe that thanks to this approach the whole sector of C1-based biomanufacturing can increase its impact within the circular economy paradigm.
References
-
Scown, C. D. Prospects for carbon-negative biomanufacturing. Trends Biotechnol. 40, 1415–1424 (2022).
Google Scholar
-
Nielsen, J., Tillegreen, C. B. & Petranovic, D. Innovation trends in industrial biotechnology. Trends Biotechnol. 40, 1160–1172 (2022).
Google Scholar
-
Clomburg, J. M., Crumbley, A. M. & Gonzalez, R. Industrial biomanufacturing: The future of chemical production. Science 355, aag0804 (2017). A perspective article proposing C1-feedstocks as promising substrates to realize sustainable and decentralized industrial biomanufacturing.
Google Scholar
-
Zhang, Y.-H. P., Sun, J. & Ma, Y. Biomanufacturing: history and perspective. J. Ind. Microbiol. Biotechnol. 44, 773–784 (2017).
Google Scholar
-
De Marco, P. Methylotrophy versus heterotrophy: a misconception. Microbiology 150, 1606–1607 (2004).
Google Scholar
-
Lee, S. Y. & Kim, H. U. Systems strategies for developing industrial microbial strains. Nat. Biotechnol. 33, 1061–1072 (2015).
Google Scholar
-
Zhu, X.-G., Long, S. P. & Ort, D. R. Improving photosynthetic efficiency for greater yield. Annu. Rev. Plant Biol. 61, 235–261 (2010).
Google Scholar
-
Zhu, X.-G., Long, S. P. & Ort, D. R. What is the maximum efficiency with which photosynthesis can convert solar energy into biomass? Curr. Opin. Biotechnol. 19, 153–159 (2008).
Google Scholar
-
Amthor, J. S. From sunlight to phytomass: on the potential efficiency of converting solar radiation to phyto-energy. N. Phytologist 188, 939–959 (2010).
Google Scholar
-
de Moraes Barbosa, A., Rebes Zilliani, R., Tiritan, C. S., Maia Souza, G. & de Almeida Silva, M. Energy conversion efficiency in sugarcane cultivars as a function of production environments in Brazil. Renew. Sustain. Energy Rev. 150, 111500 (2021).
Google Scholar
-
Campbell, B. et al. Agriculture production as a major driver of the Earth system exceeding planetary boundaries. Ecol. Soc. 22, 8 (2017).
-
Stephanopoulos, G. Challenges in engineering microbes for biofuels production. Science 315, 801–804 (2007).
Google Scholar
-
Phitsuwan, P., Sakka, K. & Ratanakhanokchai, K. Improvement of lignocellulosic biomass in planta: a review of feedstocks, biomass recalcitrance, and strategic manipulation of ideal plants designed for ethanol production and processability. Biomass-. Bioenergy 58, 390–405 (2013).
Google Scholar
-
Rasala, B. A. & Mayfield, S. P. Photosynthetic biomanufacturing in green algae; production of recombinant proteins for industrial, nutritional, and medical uses. Photosynth. Res. 123, 227–239 (2015).
Google Scholar
-
Luan, G. & Lu, X. Tailoring cyanobacterial cell factory for improved industrial properties. Biotechnol. Adv. 36, 430–442 (2018).
Google Scholar
-
Ruiz, J. et al. Towards industrial products from microalgae. Energy Environ. Sci. 9, 3036–3043 (2016).
Google Scholar
-
de Vree, J. H., Bosma, R., Janssen, M., Barbosa, M. J. & Wijffels, R. H. Comparison of four outdoor pilot-scale photobioreactors. Biotechnol. Biofuels 8, 215 (2015).
Google Scholar
-
de Vree, J. H. et al. Turbidostat operation of outdoor pilot-scale photobioreactors. Algal Res. 18, 198–208 (2016).
Google Scholar
-
Fernández, F. G. A. et al. The role of microalgae in the bioeconomy. N. Biotechnol. 61, 99–107 (2021).
Google Scholar
-
Chen, H., Li, T. & Wang, Q. Ten years of algal biofuel and bioproducts: gains and pains. Planta 249, 195–219 (2019).
Google Scholar
-
Ooms, M. D., Dinh, C. T., Sargent, E. H. & Sinton, D. Photon management for augmented photosynthesis. Nat. Commun. 7, 12699 (2016).
Google Scholar
-
Kim, J. Y., Lee, J.-W., Jung, H. S., Shin, H. & Park, N.-G. High-efficiency perovskite solar cells. Chem. Rev. 120, 7867–7918 (2020).
Google Scholar
-
Green, M. A. et al. Solar cell efficiency tables (version 62). Prog. Photovoltaics: Res. Appl. 31, 651–663 (2023).
Google Scholar
-
Gleizer, S., Bar-On, Y. M., Ben-Nissan, R. & Milo, R. Engineering microbes to produce fuel, commodities, and food from CO2. Cell Rep. Phys. Sci. 1, 100223 (2020).
Google Scholar
-
Satanowski, A. & Bar-Even, A. A one-carbon path for fixing CO2. EMBO Rep. 21, e50273 (2020).
Google Scholar
-
Claassens, N. J., Cotton, C. A. R., Kopljar, D. & Bar-Even, A. Making quantitative sense of electromicrobial production. Nat. Catal. 2, 437–447 (2019).
Google Scholar
-
Cotton, C. A., Claassens, N. J., Benito-Vaquerizo, S. & Bar-Even, A. Renewable methanol and formate as microbial feedstocks. Curr. Opin. Biotechnol. 62, 168–180 (2020).
Google Scholar
-
Stöckl, M., Claassens, N., Lindner, S., Klemm, E. & Holtmann, D. Coupling electrochemical CO2 reduction to microbial product generation – identification of the gaps and opportunities. Curr. Opin. Biotechnol. 74, 154–163 (2022).
Google Scholar
-
Gao, W. et al. Industrial carbon dioxide capture and utilization: state of the art and future challenges. Chem. Soc. Rev. 49, 8584–8686 (2020).
Google Scholar
-
Zhu, P. & Chen, X. Converting heterotrophic Escherichia coli into synthetic C1-trophic modes. Trends Chem. 4, 860–862 (2022).
Google Scholar
-
Claassens, N. J., Sousa, D. Z., dos Santos, V. A. P. M., de Vos, W. M. & van der Oost, J. Harnessing the power of microbial autotrophy. Nat. Rev. Microbiol 14, 692–706 (2016).
Google Scholar
-
Haas, T., Krause, R., Weber, R., Demler, M. & Schmid, G. Technical photosynthesis involving CO2 electrolysis and fermentation. Nat. Catal. 1, 32–39 (2018).
Google Scholar
-
Leger, D. et al. Photovoltaic-driven microbial protein production can use land and sunlight more efficiently than conventional crops. Proc. Natl Acad. Sci. USA 118, e2015025118 (2021).
Google Scholar
-
Blankenship, R. E. et al. Comparing photosynthetic and photovoltaic efficiencies and recognizing the potential for improvement. Science 332, 805–809 (2011).
Google Scholar
-
Liu, Z., Wang, K., Chen, Y., Tan, T. & Nielsen, J. Third-generation biorefineries as the means to produce fuels and chemicals from CO2. Nat. Catal. 3, 274–288 (2020).
Google Scholar
-
Jiang, W. et al. Metabolic engineering strategies to enable microbial utilization of C1 feedstocks. Nat. Chem. Biol. 17, 845–855 (2021).
Google Scholar
-
Lv, X. et al. C1-based biomanufacturing: advances, challenges and perspectives. Bioresour. Technol. 367, 128259 (2023).
Google Scholar
-
Liew, F. E. et al. Carbon-negative production of acetone and isopropanol by gas fermentation at industrial pilot scale. Nat. Biotechnol. 40, 335–344 (2022). Demonstration of a carbon negative bioprocess to produce bulk chemicals from C1 with gas fermentation.
Google Scholar
-
Lu, L. et al. CRISPR-based metabolic engineering in non-model microorganisms. Curr. Opin. Biotechnol. 75, 102698 (2022).
Google Scholar
-
Volke, D. C., Orsi, E. & Nikel, P. I. Emergent CRISPR–Cas-based technologies for engineering non-model bacteria. Curr. Opin. Microbiol. 75, 102353 (2023).
Google Scholar
-
Riley, L. A. & Guss, A. M. Approaches to genetic tool development for rapid domestication of non-model microorganisms. Biotechnol. Biofuels 14, 30 (2021).
Google Scholar
-
Fatma, Z., Schultz, J. C. & Zhao, H. Recent advances in domesticating non-model microorganisms. Biotechnol. Prog. 36, e3008 (2020).
Google Scholar
-
Gleizer, S. et al. Conversion of Escherichia coli to generate all biomass carbon from CO2. Cell 179, 1255–1263.e12 (2019). Functional implementation of a Calvin-Bensom-Bassham cycle in the model polytroph Escherichia coli to support full biomass synthesis from CO2.
Google Scholar
-
Bang, J., Hwang, C. H., Ahn, J. H., Lee, J. A. & Lee, S. Y. Escherichia coli is engineered to grow on CO2 and formic acid. Nat. Microbiol. 5, 1459–1463 (2020).
Google Scholar
-
Kim, S. et al. Growth of E. coli on formate and methanol via the reductive glycine pathway. Nat. Chem. Biol. 16, 538–545 (2020).
Google Scholar
-
Chen, F. Y.-H., Jung, H.-W., Tsuei, C.-Y. & Liao, J. C. Converting Escherichia coli to a synthetic methylotroph growing solely on methanol. Cell 182, 933–946.e14 (2020).
Google Scholar
-
Keller, P. et al. Generation of an Escherichia coli strain growing on methanol via the ribulose monophosphate cycle. Nat. Commun. 13, 5243 (2022). Engineering synthetic methylotrophy in E. coli. Evolution showed that gene expression rearrangement were necessary to enable high content of C1 fixating enzymes, as has been shown in various organisms to date.
Google Scholar
-
Wenk, S. et al. Synthetic carbon fixation via the autocatalytic serine threonine cycle. Preprint at https://doi.org/10.1101/2022.09.28.509898 (2022). Demonstration of a synthetic C1-assimilation route in vivo in Escherichia coli.
-
Chen, A. Y. & Lan, E. I. Chemical production from methanol using natural and synthetic methylotrophs. Biotechnol. J. 15, 1900356 (2020).
Google Scholar
-
Orsi, E., Beekwilder, J., Eggink, G., Kengen, S. W. M. & Weusthuis, R. A. The transition of Rhodobacter sphaeroides into a microbial cell factory. Biotechnol. Bioeng. 118, 531–541 (2021).
Google Scholar
-
Panich, J., Fong, B. & Singer, S. W. Metabolic engineering of Cupriavidus necator H16 for sustainable biofuels from CO2. Trends Biotechnol. 39, 412–424 (2021).
Google Scholar
-
Nybo, S. E., Khan, N. E., Woolston, B. M. & Curtis, W. R. Metabolic engineering in chemolithoautotrophic hosts for the production of fuels and chemicals. Metab. Eng. 30, 105–120 (2015).
Google Scholar
-
Toepel, J., Karande, R., Klähn, S. & Bühler, B. Cyanobacteria as whole-cell factories: current status and future prospectives. Curr. Opin. Biotechnol. 80, 102892 (2023).
Google Scholar
-
Bourgade, B., Minton, N. P. & Islam, M. A. Genetic and metabolic engineering challenges of C1-gas fermenting acetogenic chassis organisms. FEMS Microbiol. Rev. 45, fuab008 (2021).
Google Scholar
-
Sanford, P. A. & Woolston, B. M. Synthetic or natural? Metabolic engineering for assimilation and valorization of methanol. Curr. Opin. Biotechnol. 74, 171–179 (2022).
Google Scholar
-
Veenstra, T. D. Omics in systems biology: current progress and future outlook. PROTEOMICS 21, 2000235 (2021).
Google Scholar
-
Matthews, B. J. & Vosshall, L. B. How to turn an organism into a model organism in 10 ‘easy’ steps. J. Exp. Biol. 223, jeb218198 (2020).
Google Scholar
-
Müller, B. & Grossniklaus, U. Model organisms — A historical perspective. J. Proteom. 73, 2054–2063 (2010).
Google Scholar
-
van der Oost, J. & Patinios, C. The genome editing revolution. Trends Biotechnol. 41, 396–409 (2023).
Google Scholar
-
Knott, G. J. & Doudna, J. A. CRISPR-Cas guides the future of genetic engineering. Science 361, 866–869 (2018).
Google Scholar
-
Jinek, M. et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816–821 (2012).
Google Scholar
-
DiCarlo, J. E. et al. Genome engineering in Saccharomyces cerevisiae using CRISPR-Cas systems. Nucleic Acids Res. 41, 4336–4343 (2013).
Google Scholar
-
Donohoue, P. D., Barrangou, R. & May, A. P. Advances in industrial biotechnology using CRISPR-Cas systems. Trends Biotechnol. 36, 134–146 (2018).
Google Scholar
-
Mougiakos, I., Bosma, E. F., Ganguly, J., van der Oost, J. & van Kranenburg, R. Hijacking CRISPR-Cas for high-throughput bacterial metabolic engineering: advances and prospects. Curr. Opin. Biotechnol. 50, 146–157 (2018).
Google Scholar
-
Erb, T. J., Jones, P. R. & Bar-Even, A. Synthetic metabolism: metabolic engineering meets enzyme design. Curr. Opin. Chem. Biol. 37, 56–62 (2017).
Google Scholar
-
Martin, C. H., Nielsen, D. R., Solomon, K. V. & Prather, K. L. J. Synthetic metabolism: engineering biology at the protein and pathway scales. Chem. Biol. 16, 277–286 (2009).
Google Scholar
-
Antonovsky, N. et al. Sugar synthesis from CO2 in Escherichia coli. Cell 166, 115–125 (2016).
Google Scholar
-
Schada von Borzyskowski, L. et al. An engineered Calvin-Benson-Bassham cycle for carbon dioxide fixation in Methylobacterium extorquens AM1. Metab. Eng. 47, 423–433 (2018).
Google Scholar
-
Gassler, T. et al. The industrial yeast Pichia pastoris is converted from a heterotroph into an autotroph capable of growth on CO2. Nat. Biotechnol. 38, 210–216 (2020).
Google Scholar
-
Bar-Even, A., Noor, E., Flamholz, A. & Milo, R. Design and analysis of metabolic pathways supporting formatotrophic growth for electricity-dependent cultivation of microbes. Biochim. Biophys. Acta (BBA) – Bioenerg. 1827, 1039–1047 (2013).
Google Scholar
-
Sánchez-Andrea, I. et al. The reductive glycine pathway allows autotrophic growth of Desulfovibrio desulfuricans. Nat. Commun. 11, 5090 (2020).
Google Scholar
-
Claassens, N. J. et al. Replacing the Calvin cycle with the reductive glycine pathway in Cupriavidus necator. Metab. Eng. 62, 30–41 (2020).
Google Scholar
-
Turlin, J., Dronsella, B., De Maria, A., Lindner, S. N. & Nikel, P. I. Integrated rational and evolutionary engineering of genome-reduced Pseudomonas putida strains promotes synthetic formate assimilation. Metab. Eng. 74, 191–205 (2022).
Google Scholar
-
Bruinsma, L., Wenk, S., Claassens, N. J. & Martins dos Santos, V. A. P. Paving the way for synthetic C1 – Metabolism in Pseudomonas putida through the reductive glycine pathway. Metab. Eng. 76, 215–224 (2023).
Google Scholar
-
Yishai, O., Goldbach, L., Tenenboim, H., Lindner, S. N. & Bar-Even, A. Engineered assimilation of exogenous and endogenous formate in Escherichia coli. ACS Synth. Biol. 6, 1722–1731 (2017).
Google Scholar
-
Yishai, O., Bouzon, M., Döring, V. & Bar-Even, A. In vivo assimilation of one-carbon via a synthetic reductive glycine pathway in Escherichia coli. ACS Synth. Biol. 7, 2023–2028 (2018).
Google Scholar
-
Gonzalez de la Cruz, J., Machens, F., Messerschmidt, K. & Bar-Even, A. Core catalysis of the reductive glycine pathway demonstrated in yeast. ACS Synth. Biol. 8, 911–917 (2019).
Google Scholar
-
Bennett, R. K. et al. Engineering Escherichia coli for methanol-dependent growth on glucose for metabolite production. Metab. Eng. 60, 45–55 (2020).
Google Scholar
-
Müller, J. E. N. et al. Engineering Escherichia coli for methanol conversion. Metab. Eng. 28, 190–201 (2015).
Google Scholar
-
Chen, C.-T. et al. Synthetic methanol auxotrophy of Escherichia coli for methanol-dependent growth and production. Metab. Eng. 49, 257–266 (2018).
Google Scholar
-
Keller, P. et al. Methanol-dependent Escherichia coli strains with a complete ribulose monophosphate cycle. Nat. Commun. 11, 5403 (2020).
Google Scholar
-
Schwander, T., Schada von Borzyskowski, L., Burgener, S., Cortina, N. S. & Erb, T. J. A synthetic pathway for the fixation of carbon dioxide in vitro. Science 354, 900–904 (2016). First in vitro demonstration of a synthetic pathway for CO2 fixation.
Google Scholar
-
Luo, S. et al. A cell-free self-replenishing CO2-fixing system. Nat. Catal. 5, 154–162 (2022).
Google Scholar
-
McLean, R. et al. Exploring alternative pathways for the in vitro establishment of the HOPAC cycle for synthetic CO2 fixation. Sci. Adv. 9, eadh4299 (2023).
Google Scholar
-
Formate Assimilation: The Metabolic Architecture of Natural and Synthetic Pathways | Biochemistry. https://pubs.acs.org/doi/10.1021/acs.biochem.6b00495.
-
Ben Nissan, R. et al. Autotrophic growth of E. coli is achieved by a small number of genetic changes. Preprint at bioRxiv https://doi.org/10.1101/2023.06.03.543535 (2023). Determination of the minimal sets mutations necessary to transform E. coli into an autotrophic organism matching the heavily evolved strain from reference 43.
-
Hendrickson, E. L. et al. Expressed genome of Methylobacillus flagellatus as defined through comprehensive proteomics and new insights into methylotrophy. J. Bacteriol. 192, 4859–4867 (2010).
Google Scholar
-
Collas, F. et al. Engineering the biological conversion of formate into crotonate in Cupriavidus necator. Metab. Eng. 79, 49–65 (2023).
Google Scholar
-
Kim, S. et al. Optimizing E. coli as a formatotrophic platform for bioproduction via the reductive glycine pathway. Front. Bioeng. Biotechnol. 11, 1091899 (2023).
-
Barenholz, U. et al. Design principles of autocatalytic cycles constrain enzyme kinetics and force low substrate saturation at flux branch points. eLife 6, e20667 (2017).
Google Scholar
-
Herz, E. et al. The genetic basis for the adaptation of E. coli to sugar synthesis from CO2. Nat. Commun. 8, 1705 (2017).
Google Scholar
-
Flamholz, A. I. et al. Functional reconstitution of a bacterial CO2 concentrating mechanism in Escherichia coli. eLife 9, e59882 (2020).
Google Scholar
-
Sun, Y. et al. Decoding the absolute stoichiometric composition and structural plasticity of α-carboxysomes. mBio 13, e03629–21 (2022).
Google Scholar
-
Chen, T. et al. Incorporation of functional rubisco activases into engineered carboxysomes to enhance carbon fixation. ACS Synth. Biol. 11, 154–161 (2022).
Google Scholar
-
Flamholz, A. I. et al. Trajectories for the evolution of bacterial CO2-concentrating mechanisms. Proc. Natl Acad. Sci. USA 119, e2210539119 (2022).
Google Scholar
-
Chen, T. et al. Engineering α-carboxysomes into plant chloroplasts to support autotrophic photosynthesis. Nat. Commun. 14, 2118 (2023).
Google Scholar
-
Claassens, N. J. et al. Engineering the Reductive Glycine Pathway: A Promising Synthetic Metabolism Approach for C1-Assimilation. in One-Carbon Feedstocks for Sustainable Bioproduction (eds. Zeng, A.-P. & Claassens, N. J.) 299–350 (Springer International Publishing, 2022). https://doi.org/10.1007/10_2021_181.
-
Dronsella, B. et al. Engineered synthetic one-carbon fixation exceeds yield of the Calvin Cycle. Preprint at https://doi.org/10.1101/2022.10.19.512895 (2022). Replacement of the Calvin-Bensom-Bassham cycle with a genome integrated reductive glycine pathway in the C1-trophic bacterium Cupriavidus necator results in improved biomass yield on formate
-
Orsi, E., Claassens, N. J., Nikel, P. I. & Lindner, S. N. Optimizing microbial networks through metabolic bypasses. Biotechnol. Adv. 60, 108035 (2022).
Google Scholar
-
Okamura, E., Tomita, T., Sawa, R., Nishiyama, M. & Kuzuyama, T. Unprecedented acetoacetyl-coenzyme A synthesizing enzyme of the thiolase superfamily involved in the mevalonate pathway. Proc. Natl Acad. Sci. USA 107, 11265–11270 (2010).
Google Scholar
-
Lan, E. I. & Liao, J. C. ATP drives direct photosynthetic production of 1-butanol in cyanobacteria. Proc. Natl Acad. Sci. USA 109, 6018–6023 (2012).
Google Scholar
-
Cain, A. K. et al. A decade of advances in transposon-insertion sequencing. Nat. Rev. Genet 21, 526–540 (2020).
Google Scholar
-
Jahn, M. et al. Protein allocation and utilization in the versatile chemolithoautotroph Cupriavidus necator. eLife 10, e69019 (2021). Systems level investigation of the proteome allocation in the C1-trophic bacterium Cupriavidus necator sheds light on the reallocation of protein resources under different growth conditions.
Google Scholar
-
Pearcy, N. et al. A genome-scale metabolic model of Cupriavidus necator H16 integrated with TraDIS and transcriptomic data reveals metabolic insights for biotechnological applications. PLoS Computational Biol. 18, e1010106 (2022).
Google Scholar
-
Woods, C. et al. Required gene set for autotrophic growth of Clostridium autoethanogenum. Appl. Environ. Microbiol. 88, e02479–21 (2022).
Google Scholar
-
Donati, S. et al. Multi-omics analysis of CRISPRi-knockdowns identifies mechanisms that buffer decreases of enzymes in E. coli metabolism. Cell Syst. 12, 56–67.e6 (2021).
Google Scholar
-
Peters, J. M. et al. A comprehensive, CRISPR-based functional analysis of essential genes in bacteria. Cell 165, 1493–1506 (2016).
Google Scholar
-
Cui, L. et al. A CRISPRi screen in E. coli reveals sequence-specific toxicity of dCas9. Nat. Commun. 9, 1912 (2018).
-
Shin, J. et al. Genome-wide CRISPRi screen identifies enhanced autolithotrophic phenotypes in acetogenic bacterium Eubacterium limosum. Proc. Natl Acad. Sci. USA 120, e2216244120 (2023).
Google Scholar
-
Valgepea, K. et al. Absolute proteome quantification in the gas-fermenting acetogen Clostridium autoethanogenum. mSystems 7, e00026–22 (2022). Resource allocation study showing that 30% of the C. autoethanogenum proteome is allocated towards C1 fixation.
Google Scholar
-
Valgepea, K. et al. Maintenance of ATP homeostasis triggers metabolic shifts in gas-fermenting acetogens. cels 4, 505–515.e5 (2017).
Google Scholar
-
Mahamkali, V. et al. Redox controls metabolic robustness in the gas-fermenting acetogen Clostridium autoethanogenum. Proc. Natl Acad. Sci. USA 117, 13168–13175 (2020).
Google Scholar
-
Müller, A. L. et al. An alternative resource allocation strategy in the chemolithoautotrophic archaeon Methanococcus maripaludis. Proc. Natl Acad. Sci. USA 118, e2025854118 (2021).
Google Scholar
-
Hui, S. et al. Quantitative proteomic analysis reveals a simple strategy of global resource allocation in bacteria. Mol. Syst. Biol. 11, e784–e784 (2015).
Google Scholar
-
Shimizu, R. et al. New insight into the role of the calvin cycle: reutilization of CO2 emitted through sugar degradation. Sci. Rep. 5, 11617 (2015).
Google Scholar
-
Alagesan, S., Minton, N. P. & Malys, N. 13C-assisted metabolic flux analysis to investigate heterotrophic and mixotrophic metabolism in Cupriavidus necator H16. Metabolomics 14, 9 (2017).
Google Scholar
-
Fukui, T. et al. Metabolite profiles of polyhydroxyalkanoate-producing Ralstonia eutropha H16. Metabolomics 10, 190–202 (2014).
Google Scholar
-
Rychel, K. et al. iModulonDB: a knowledgebase of microbial transcriptional regulation derived from machine learning. Nucleic Acids Res. 49, D112–D120 (2021).
Google Scholar
-
de Souza Pinto Lemgruber, R. et al. Quantitative analysis of tetrahydrofolate metabolites from clostridium autoethanogenum. Metabolomics 14, 35 (2018).
Google Scholar
-
Schittmayer, M., Birner-Gruenberger, R. & Zamboni, N. Quantification of cellular folate species by LC-MS after stabilization by derivatization. Anal. Chem. 90, 7349–7356 (2018).
Google Scholar
-
Lempp, M. et al. Systematic identification of metabolites controlling gene expression in E. coli. Nat. Commun. 10, 4463 (2019).
Google Scholar
-
Radoš, D., Donati, S., Lempp, M., Rapp, J. & Link, H. Homeostasis of the biosynthetic E. coli metabolome. iScience 25, 104503 (2022).
Google Scholar
-
Donati, S., Sander, T. & Link, H. Crosstalk between transcription and metabolism: how much enzyme is enough for a cell? Wiley Interdiscip. Rev.: Syst. Biol. Med. 10, e1396 (2018).
-
Diether, M., Nikolaev, Y., Allain, F. H. & Sauer, U. Systematic mapping of protein‐metabolite interactions in central metabolism of Escherichia coli. Mol. Syst. Biol. 15, e9008 (2019).
-
Piazza, I. et al. A map of protein-metabolite interactions reveals principles of chemical communication. Cell 172, 358–372.e23 (2018).
Google Scholar
-
Sporre, E. et al. Metabolite interactions in the bacterial Calvin cycle and implications for flux regulation. Commun. Biol. 6, 947 (2023).
-
Lu, K.-J. et al. An ATP-sensitive phosphoketolase regulates carbon fixation in cyanobacteria. Nat. Metab. 5, 1111–1126 (2023).
Google Scholar
-
Arfman, N. et al. Methanol metabolism in thermotolerant methylotrophic Bacillus strains involving a novel catabolic NAD-dependent methanol dehydrogenase as a key enzyme. Arch. Microbiol. 152, 280–288 (1989).
Google Scholar
-
Straathof, A. J. J. et al. Grand research challenges for sustainable industrial biotechnology. Trends Biotechnol. 37, 1042–1050 (2019).
Google Scholar
-
Baumschabl, M. et al. Conversion of CO2 into organic acids by engineered autotrophic yeast. Proc. Natl Acad. Sci. USA 119, e2211827119 (2022).
Google Scholar
-
Turlin, J., Puiggené, Ò., Donati, S., Wirth, N. T. & Nikel, P. I. Core and auxiliary functions of one-carbon metabolism in Pseudomonas putida exposed by a systems-level analysis of transcriptional and physiological responses. mSystems 8, e00004–e00023 (2023).
Google Scholar
-
Santomartino, R. et al. Toward sustainable space exploration: a roadmap for harnessing the power of microorganisms. Nat. Commun. 14, 1391 (2023).
Google Scholar
-
Berliner, A. J. et al. Towards a Biomanufactory on Mars. Front. Astron. Space Sci. 8, 711550 (2021).
-
Nangle, S. N. et al. The case for biotech on Mars. Nat. Biotechnol. 38, 401–407 (2020).
Google Scholar
-
Marquez-Zavala, E. & Utrilla, J. Engineering resource allocation in artificially minimized cells: is genome reduction the best strategy? Microb. Biotechnol. 16, 990–999 (2023).
Google Scholar
-
Xu, X. et al. Trimming the genomic fat: minimising and re-functionalising genomes using synthetic biology. Nat. Commun. 14, 1984 (2023).
Google Scholar
-
Calvey, C. H. et al. Improving growth of Cupriavidus necator H16 on formate using adaptive laboratory evolution-informed engineering. Metab. Eng. 75, 78–90 (2023). Genome reduction combined with adaptive laboratory evolution improve growth rate and yield in a natural C1-trophic bacterium.
Google Scholar
Acknowledgements
This work was supported by The Novo Nordisk Foundation (NNF20CC0035580). E.O. was supported by the European Union’s Horizon 2020 Research and Innovation Program under the Marie Skłodowska-Curie grant agreement No. 101065339 (ROAD). The financial support from The Novo Nordisk Foundation through grants LiFe (NNF18OC0034818) and TARGET (NNF21OC0067996), the Danish Council for Independent Research (SWEET, DFF-Research Project 8021-00039B), and the European Union’s Horizon 2020 Research and Innovation Programme under grant agreement No. 814418 (SinFonia) to P.I.N. is likewise gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
E.O. and S.D. conceived and co-wrote the manuscript. P.I.N. and L.K.N. provided extensive feedback. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Source Data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
Reprints and Permissions
About this article
Cite this article
Orsi, E., Nikel, P.I., Nielsen, L.K. et al. Synergistic investigation of natural and synthetic C1-trophic microorganisms to foster a circular carbon economy.
Nat Commun 14, 6673 (2023). https://doi.org/10.1038/s41467-023-42166-w
-
Received: 04 August 2023
-
Accepted: 02 October 2023
-
Published: 21 October 2023
-
DOI: https://doi.org/10.1038/s41467-023-42166-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.