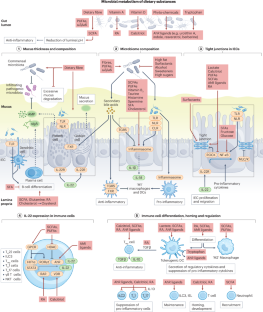
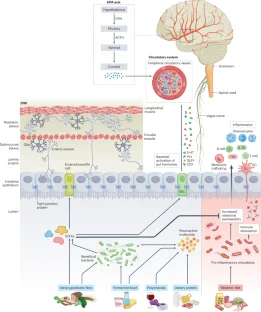
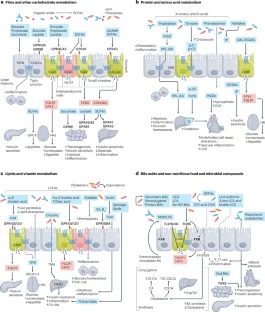
Abstract
The gut microbiome has an undeniable role in mediating the health effects of the diet, given its ability to co-digest nutrients and influence nutrient signalling to multiple organ systems. As a suboptimal diet is a major risk factor for and contributor to disease, understanding the multidirectional interactions between the food we eat, the gut microbiome and the different body organ systems is crucial from a public health perspective. Indeed, this research area is leading to the refinement of nutritional concepts and strategies to optimize health through diet. In this Review, we provide an update on how dietary patterns and food intake shape gut microbiome features, the mode of action of diet–microorganism interactions on the immune, nervous and cardiometabolic systems and how this knowledge could explain the heterogeneity of dietary responses, and support food-based dietary guidelines and medical and precision nutrition. Finally, we discuss the knowledge gaps and research efforts needed to progress towards the integration of microbiome science with more precise dietary advice to leverage the role of nutrition in human health.
Key points
-
Exploring the granularity of the diet and multidimensional aspects of foods together with advanced big data-driven analyses offers opportunities to better capture diet–microbiome–health relationships and explain unsolved response patterns and mechanisms in humans.
-
Microbially produced metabolites from dietary sources and microbial cell structural constituents have been demonstrated to regulate immune, endocrine and nervous system functions, supporting their causal role in diverse health domains.
-
Current food-based dietary guidelines are not yet microbiome-oriented but generally provide advice for maintaining diet–microorganism synergies relevant to human health.
-
Further understanding of the contribution of the gut microbiome to the heterogeneity of diet-related responses is vital to optimizing the role of nutrition in public health.
-
The gut microbiome contributes to postprandial responses to meals, suggesting the potential to predict the long-term health consequences of our diet more accurately.
-
The response to the diet and the links to the gut microbiome vary in health and disease contexts and need to be scrutinized for the development of more precise nutritional practices.
This is a preview of subscription content, access via your institution
Access options
/* style specs end */
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
/* style specs start */
style {
display: none !important;
}
.LiveAreaSection * {
align-content: stretch;
align-items: stretch;
align-self: auto;
animation-delay: 0s;
animation-direction: normal;
animation-duration: 0s;
animation-fill-mode: none;
animation-iteration-count: 1;
animation-name: none;
animation-play-state: running;
animation-timing-function: ease;
azimuth: center;
backface-visibility: visible;
background-attachment: scroll;
background-blend-mode: normal;
background-clip: borderBox;
background-color: transparent;
background-image: none;
background-origin: paddingBox;
background-position: 0 0;
background-repeat: repeat;
background-size: auto auto;
block-size: auto;
border-block-end-color: currentcolor;
border-block-end-style: none;
border-block-end-width: medium;
border-block-start-color: currentcolor;
border-block-start-style: none;
border-block-start-width: medium;
border-bottom-color: currentcolor;
border-bottom-left-radius: 0;
border-bottom-right-radius: 0;
border-bottom-style: none;
border-bottom-width: medium;
border-collapse: separate;
border-image-outset: 0s;
border-image-repeat: stretch;
border-image-slice: 100%;
border-image-source: none;
border-image-width: 1;
border-inline-end-color: currentcolor;
border-inline-end-style: none;
border-inline-end-width: medium;
border-inline-start-color: currentcolor;
border-inline-start-style: none;
border-inline-start-width: medium;
border-left-color: currentcolor;
border-left-style: none;
border-left-width: medium;
border-right-color: currentcolor;
border-right-style: none;
border-right-width: medium;
border-spacing: 0;
border-top-color: currentcolor;
border-top-left-radius: 0;
border-top-right-radius: 0;
border-top-style: none;
border-top-width: medium;
bottom: auto;
box-decoration-break: slice;
box-shadow: none;
box-sizing: border-box;
break-after: auto;
break-before: auto;
break-inside: auto;
caption-side: top;
caret-color: auto;
clear: none;
clip: auto;
clip-path: none;
color: initial;
column-count: auto;
column-fill: balance;
column-gap: normal;
column-rule-color: currentcolor;
column-rule-style: none;
column-rule-width: medium;
column-span: none;
column-width: auto;
content: normal;
counter-increment: none;
counter-reset: none;
cursor: auto;
display: inline;
empty-cells: show;
filter: none;
flex-basis: auto;
flex-direction: row;
flex-grow: 0;
flex-shrink: 1;
flex-wrap: nowrap;
float: none;
font-family: initial;
font-feature-settings: normal;
font-kerning: auto;
font-language-override: normal;
font-size: medium;
font-size-adjust: none;
font-stretch: normal;
font-style: normal;
font-synthesis: weight style;
font-variant: normal;
font-variant-alternates: normal;
font-variant-caps: normal;
font-variant-east-asian: normal;
font-variant-ligatures: normal;
font-variant-numeric: normal;
font-variant-position: normal;
font-weight: 400;
grid-auto-columns: auto;
grid-auto-flow: row;
grid-auto-rows: auto;
grid-column-end: auto;
grid-column-gap: 0;
grid-column-start: auto;
grid-row-end: auto;
grid-row-gap: 0;
grid-row-start: auto;
grid-template-areas: none;
grid-template-columns: none;
grid-template-rows: none;
height: auto;
hyphens: manual;
image-orientation: 0deg;
image-rendering: auto;
image-resolution: 1dppx;
ime-mode: auto;
inline-size: auto;
isolation: auto;
justify-content: flexStart;
left: auto;
letter-spacing: normal;
line-break: auto;
line-height: normal;
list-style-image: none;
list-style-position: outside;
list-style-type: disc;
margin-block-end: 0;
margin-block-start: 0;
margin-bottom: 0;
margin-inline-end: 0;
margin-inline-start: 0;
margin-left: 0;
margin-right: 0;
margin-top: 0;
mask-clip: borderBox;
mask-composite: add;
mask-image: none;
mask-mode: matchSource;
mask-origin: borderBox;
mask-position: 0 0;
mask-repeat: repeat;
mask-size: auto;
mask-type: luminance;
max-height: none;
max-width: none;
min-block-size: 0;
min-height: 0;
min-inline-size: 0;
min-width: 0;
mix-blend-mode: normal;
object-fit: fill;
object-position: 50% 50%;
offset-block-end: auto;
offset-block-start: auto;
offset-inline-end: auto;
offset-inline-start: auto;
opacity: 1;
order: 0;
orphans: 2;
outline-color: initial;
outline-offset: 0;
outline-style: none;
outline-width: medium;
overflow: visible;
overflow-wrap: normal;
overflow-x: visible;
overflow-y: visible;
padding-block-end: 0;
padding-block-start: 0;
padding-bottom: 0;
padding-inline-end: 0;
padding-inline-start: 0;
padding-left: 0;
padding-right: 0;
padding-top: 0;
page-break-after: auto;
page-break-before: auto;
page-break-inside: auto;
perspective: none;
perspective-origin: 50% 50%;
pointer-events: auto;
position: static;
quotes: initial;
resize: none;
right: auto;
ruby-align: spaceAround;
ruby-merge: separate;
ruby-position: over;
scroll-behavior: auto;
scroll-snap-coordinate: none;
scroll-snap-destination: 0 0;
scroll-snap-points-x: none;
scroll-snap-points-y: none;
scroll-snap-type: none;
shape-image-threshold: 0;
shape-margin: 0;
shape-outside: none;
tab-size: 8;
table-layout: auto;
text-align: initial;
text-align-last: auto;
text-combine-upright: none;
text-decoration-color: currentcolor;
text-decoration-line: none;
text-decoration-style: solid;
text-emphasis-color: currentcolor;
text-emphasis-position: over right;
text-emphasis-style: none;
text-indent: 0;
text-justify: auto;
text-orientation: mixed;
text-overflow: clip;
text-rendering: auto;
text-shadow: none;
text-transform: none;
text-underline-position: auto;
top: auto;
touch-action: auto;
transform: none;
transform-box: borderBox;
transform-origin: 50% 50%0;
transform-style: flat;
transition-delay: 0s;
transition-duration: 0s;
transition-property: all;
transition-timing-function: ease;
vertical-align: baseline;
visibility: visible;
white-space: normal;
widows: 2;
width: auto;
will-change: auto;
word-break: normal;
word-spacing: normal;
word-wrap: normal;
writing-mode: horizontalTb;
z-index: auto;
-webkit-appearance: none;
-moz-appearance: none;
-ms-appearance: none;
appearance: none;
margin: 0;
}
.LiveAreaSection {
width: 100%;
}
.LiveAreaSection .login-option-buybox {
display: block;
width: 100%;
font-size: 17px;
line-height: 30px;
color: #222;
padding-top: 30px;
font-family: Harding, Palatino, serif;
}
.LiveAreaSection .additional-access-options {
display: block;
font-weight: 700;
font-size: 17px;
line-height: 30px;
color: #222;
font-family: Harding, Palatino, serif;
}
.LiveAreaSection .additional-login > li:not(:first-child)::before {
transform: translateY(-50%);
content: “”;
height: 1rem;
position: absolute;
top: 50%;
left: 0;
border-left: 2px solid #999;
}
.LiveAreaSection .additional-login > li:not(:first-child) {
padding-left: 10px;
}
.LiveAreaSection .additional-login > li {
display: inline-block;
position: relative;
vertical-align: middle;
padding-right: 10px;
}
.BuyBoxSection {
display: flex;
flex-wrap: wrap;
flex: 1;
flex-direction: row-reverse;
margin: -30px -15px 0;
}
.BuyBoxSection .box-inner {
width: 100%;
height: 100%;
padding: 30px 5px;
display: flex;
flex-direction: column;
justify-content: space-between;
}
.BuyBoxSection p {
margin: 0;
}
.BuyBoxSection .readcube-buybox {
background-color: #f3f3f3;
flex-shrink: 1;
flex-grow: 1;
flex-basis: 255px;
background-clip: content-box;
padding: 0 15px;
margin-top: 30px;
}
.BuyBoxSection .subscribe-buybox {
background-color: #f3f3f3;
flex-shrink: 1;
flex-grow: 4;
flex-basis: 300px;
background-clip: content-box;
padding: 0 15px;
margin-top: 30px;
}
.BuyBoxSection .subscribe-buybox-nature-plus {
background-color: #f3f3f3;
flex-shrink: 1;
flex-grow: 4;
flex-basis: 100%;
background-clip: content-box;
padding: 0 15px;
margin-top: 30px;
}
.BuyBoxSection .title-readcube,
.BuyBoxSection .title-buybox {
display: block;
margin: 0;
margin-right: 10%;
margin-left: 10%;
font-size: 24px;
line-height: 32px;
color: #222;
text-align: center;
font-family: Harding, Palatino, serif;
}
.BuyBoxSection .title-asia-buybox {
display: block;
margin: 0;
margin-right: 5%;
margin-left: 5%;
font-size: 24px;
line-height: 32px;
color: #222;
text-align: center;
font-family: Harding, Palatino, serif;
}
.BuyBoxSection .asia-link,
.Link-328123652,
.Link-2926870917,
.Link-2291679238,
.Link-595459207 {
color: #069;
cursor: pointer;
text-decoration: none;
font-size: 1.05em;
font-family: -apple-system, BlinkMacSystemFont, “Segoe UI”, Roboto,
Oxygen-Sans, Ubuntu, Cantarell, “Helvetica Neue”, sans-serif;
line-height: 1.05em6;
}
.BuyBoxSection .access-readcube {
display: block;
margin: 0;
margin-right: 10%;
margin-left: 10%;
font-size: 14px;
color: #222;
padding-top: 10px;
text-align: center;
font-family: -apple-system, BlinkMacSystemFont, “Segoe UI”, Roboto,
Oxygen-Sans, Ubuntu, Cantarell, “Helvetica Neue”, sans-serif;
line-height: 20px;
}
.BuyBoxSection ul {
margin: 0;
}
.BuyBoxSection .link-usp {
display: list-item;
margin: 0;
margin-left: 20px;
padding-top: 6px;
list-style-position: inside;
}
.BuyBoxSection .link-usp span {
font-size: 14px;
color: #222;
font-family: -apple-system, BlinkMacSystemFont, “Segoe UI”, Roboto,
Oxygen-Sans, Ubuntu, Cantarell, “Helvetica Neue”, sans-serif;
line-height: 20px;
}
.BuyBoxSection .access-asia-buybox {
display: block;
margin: 0;
margin-right: 5%;
margin-left: 5%;
font-size: 14px;
color: #222;
padding-top: 10px;
text-align: center;
font-family: -apple-system, BlinkMacSystemFont, “Segoe UI”, Roboto,
Oxygen-Sans, Ubuntu, Cantarell, “Helvetica Neue”, sans-serif;
line-height: 20px;
}
.BuyBoxSection .access-buybox {
display: block;
margin: 0;
margin-right: 10%;
margin-left: 10%;
font-size: 14px;
color: #222;
opacity: 0.8px;
padding-top: 10px;
text-align: center;
font-family: -apple-system, BlinkMacSystemFont, “Segoe UI”, Roboto,
Oxygen-Sans, Ubuntu, Cantarell, “Helvetica Neue”, sans-serif;
line-height: 20px;
}
.BuyBoxSection .price-buybox {
display: block;
font-size: 30px;
color: #222;
font-family: -apple-system, BlinkMacSystemFont, “Segoe UI”, Roboto,
Oxygen-Sans, Ubuntu, Cantarell, “Helvetica Neue”, sans-serif;
padding-top: 30px;
text-align: center;
}
.BuyBoxSection .price-buybox-to {
display: block;
font-size: 30px;
color: #222;
font-family: -apple-system, BlinkMacSystemFont, “Segoe UI”, Roboto,
Oxygen-Sans, Ubuntu, Cantarell, “Helvetica Neue”, sans-serif;
text-align: center;
}
.BuyBoxSection .price-info-text {
font-size: 16px;
padding-right: 10px;
color: #222;
font-family: -apple-system, BlinkMacSystemFont, “Segoe UI”, Roboto,
Oxygen-Sans, Ubuntu, Cantarell, “Helvetica Neue”, sans-serif;
}
.BuyBoxSection .price-value {
font-size: 30px;
font-family: -apple-system, BlinkMacSystemFont, “Segoe UI”, Roboto,
Oxygen-Sans, Ubuntu, Cantarell, “Helvetica Neue”, sans-serif;
}
.BuyBoxSection .price-per-period {
font-family: -apple-system, BlinkMacSystemFont, “Segoe UI”, Roboto,
Oxygen-Sans, Ubuntu, Cantarell, “Helvetica Neue”, sans-serif;
}
.BuyBoxSection .price-from {
font-size: 14px;
padding-right: 10px;
color: #222;
font-family: -apple-system, BlinkMacSystemFont, “Segoe UI”, Roboto,
Oxygen-Sans, Ubuntu, Cantarell, “Helvetica Neue”, sans-serif;
line-height: 20px;
}
.BuyBoxSection .issue-buybox {
display: block;
font-size: 13px;
text-align: center;
color: #222;
font-family: -apple-system, BlinkMacSystemFont, “Segoe UI”, Roboto,
Oxygen-Sans, Ubuntu, Cantarell, “Helvetica Neue”, sans-serif;
line-height: 19px;
}
.BuyBoxSection .no-price-buybox {
display: block;
font-size: 13px;
line-height: 18px;
text-align: center;
padding-right: 10%;
padding-left: 10%;
padding-bottom: 20px;
padding-top: 30px;
color: #222;
font-family: -apple-system, BlinkMacSystemFont, “Segoe UI”, Roboto,
Oxygen-Sans, Ubuntu, Cantarell, “Helvetica Neue”, sans-serif;
}
.BuyBoxSection .vat-buybox {
display: block;
margin-top: 5px;
margin-right: 20%;
margin-left: 20%;
font-size: 11px;
color: #222;
padding-top: 10px;
padding-bottom: 15px;
text-align: center;
font-family: -apple-system, BlinkMacSystemFont, “Segoe UI”, Roboto,
Oxygen-Sans, Ubuntu, Cantarell, “Helvetica Neue”, sans-serif;
line-height: 17px;
}
.BuyBoxSection .tax-buybox {
display: block;
width: 100%;
color: #222;
padding: 20px 16px;
text-align: center;
font-family: -apple-system, BlinkMacSystemFont, “Segoe UI”, Roboto,
Oxygen-Sans, Ubuntu, Cantarell, “Helvetica Neue”, sans-serif;
line-height: NaNpx;
}
.BuyBoxSection .button-container {
display: flex;
padding-right: 20px;
padding-left: 20px;
justify-content: center;
}
.BuyBoxSection .button-container > * {
flex: 1px;
}
.BuyBoxSection .button-container > a:hover,
.Button-505204839:hover,
.Button-1078489254:hover,
.Button-2737859108:hover {
text-decoration: none;
}
.BuyBoxSection .btn-secondary {
background: #fff;
}
.BuyBoxSection .button-asia {
background: #069;
border: 1px solid #069;
border-radius: 0;
cursor: pointer;
display: block;
padding: 9px;
outline: 0;
text-align: center;
text-decoration: none;
min-width: 80px;
margin-top: 75px;
}
.BuyBoxSection .button-label-asia,
.ButtonLabel-3869432492,
.ButtonLabel-3296148077,
.ButtonLabel-1636778223 {
display: block;
color: #fff;
font-size: 17px;
line-height: 20px;
font-family: -apple-system, BlinkMacSystemFont, “Segoe UI”, Roboto,
Oxygen-Sans, Ubuntu, Cantarell, “Helvetica Neue”, sans-serif;
text-align: center;
text-decoration: none;
cursor: pointer;
}
.Button-505204839,
.Button-1078489254,
.Button-2737859108 {
background: #069;
border: 1px solid #069;
border-radius: 0;
cursor: pointer;
display: block;
padding: 9px;
outline: 0;
text-align: center;
text-decoration: none;
min-width: 80px;
max-width: 320px;
margin-top: 20px;
}
.Button-505204839 .btn-secondary-label,
.Button-1078489254 .btn-secondary-label,
.Button-2737859108 .btn-secondary-label {
color: #069;
}
.uList-2102244549 {
list-style: none;
padding: 0;
margin: 0;
}
/* style specs end */

References
-
GBD 2021 Risk Factors Collaborators. Global burden and strength of evidence for 88 risk factors in 204 countries and 811 subnational locations, 1990-2021: a systematic analysis for the Global Burden of Disease Study 2021. Lancet 403, 2162–2203 (2024).
Google Scholar
-
Willett, W. et al. Food in the anthropocene: the EAT-Lancet Commission on healthy diets from sustainable food systems. Lancet 393, 447–492 (2019).
Google Scholar
-
Mozaffarian, D., Callahan, E. A., Glickman, D. & Maitin-Shepard, M. Developing a national nutrition policy strategy to advance cardiometabolic health and health equity. Cell Metab. 36, 651–654 (2024).
Google Scholar
-
EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA) Scientific opinion on establishing food‐based dietary guidelines. EFSA J. 8, 1460 (2010).
-
Gkouskou, K. K. et al. A genomics perspective of personalized prevention and management of obesity. Hum. Genomics 18, 4 (2024).
Google Scholar
-
Sanz, Y. et al. Towards microbiome-informed dietary recommendations for promoting metabolic and mental health: opinion papers of the MyNewGut project. Clin. Nutr. 37, 2191–2197 (2018).
Google Scholar
-
Chadaideh, K. S. & Carmody, R. N. Host-microbial interactions in the metabolism of different dietary fats. Cell Metab. 33, 857–872 (2021).
Google Scholar
-
Perler, B. K., Friedman, E. S. & Wu, G. D. The role of the gut microbiota in the relationship between diet and human health. Annu. Rev. Physiol. 85, 449–468 (2023).
Google Scholar
-
Wolter, M. et al. Leveraging diet to engineer the gut microbiome. Nat. Rev. Gastroenterol. Hepatol. 18, 885–902 (2021).
Google Scholar
-
Fan, Y. & Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 19, 55–71 (2021).
Google Scholar
-
Carmody, R. N., Varady, K. & Turnbaugh, P. J. Digesting the complex metabolic effects of diet on the host and microbiome. Cell 187, 3857–3876 (2024).
Google Scholar
-
Kolodziejczyk, A. A., Zheng, D. & Elinav, E. Diet-microbiota interactions and personalized nutrition. Nat. Rev. Microbiol. 17, 742–753 (2019).
Google Scholar
-
Wingrove, K., Lawrence, M. A. & McNaughton, S. A. A systematic review of the methods used to assess and report dietary patterns. Front. Nutr. 9, 892351 (2022).
Google Scholar
-
Carson, T. L. et al. Rationale and study protocol for a randomized controlled feeding study to determine the structural- and functional-level effects of diet-specific interventions on the gut microbiota of non-Hispanic black and white adults. Contemp. Clin. Trials 123, 106968 (2022).
Google Scholar
-
Bowyer, R. C. E. et al. Use of dietary indices to control for diet in human gut microbiota studies. Microbiome 6, 77 (2018).
Google Scholar
-
Xiao, C. et al. Associations of dietary diversity with the gut microbiome, fecal metabolites, and host metabolism: results from 2 prospective Chinese cohorts. Am. J. Clin. Nutr. 116, 1049–1058 (2022).
Google Scholar
-
Asnicar, F. et al. Microbiome connections with host metabolism and habitual diet from 1,098 deeply phenotyped individuals. Nat. Med. 27, 321–332 (2021).
Google Scholar
-
Choi, Y., Hoops, S. L., Thoma, C. J. & Johnson, A. J. A guide to dietary pattern–microbiome data integration. J. Nutr. 152, 1187–1199 (2022).
Google Scholar
-
Cotillard, A. et al. A posteriori dietary patterns better explain variations of the gut microbiome than individual markers in the American Gut Project. Am. J. Clin. Nutr. 115, 432–443 (2022).
Google Scholar
-
Kase, B. E. et al. The development and evaluation of a literature-based dietary index for gut microbiota. Nutrients 16, 1045 (2024).
Google Scholar
-
Tagliamonte, S. et al. Relationships between diet and gut microbiome in an Italian and Dutch cohort: does the dietary protein to fiber ratio play a role? Eur. J. Nutr. 63, 741–750 (2024).
Google Scholar
-
Wu, G. D. et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 334, 105–108 (2011).
Google Scholar
-
David, L. A. et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 505, 559–563 (2014).
Google Scholar
-
O’Keefe, S. J. D. et al. Fat, fibre and cancer risk in African Americans and rural Africans. Nat. Commun. 6, 6342 (2015).
Google Scholar
-
Ghosh, T. S. et al. Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: the NU-AGE 1-year dietary intervention across five European countries. Gut 69, 1218–1228 (2020).
Google Scholar
-
Fragiadakis, G. K. et al. Long-term dietary intervention reveals resilience of the gut microbiota despite changes in diet and weight. Am. J. Clin. Nutr. 111, 1127–1136 (2020).
Google Scholar
-
Muralidharan, J. et al. Effect on gut microbiota of a 1-y lifestyle intervention with Mediterranean diet compared with energy-reduced Mediterranean diet and physical activity promotion: PREDIMED-plus study. Am. J. Clin. Nutr. 114, 1148–1158 (2021).
Google Scholar
-
Bermingham, K. M. et al. Effects of a personalized nutrition program on cardiometabolic health: a randomized controlled trial. Nat. Med. 30, 1888–1897 (2024).
Google Scholar
-
Wan, Y. et al. Effects of dietary fat on gut microbiota and faecal metabolites, and their relationship with cardiometabolic risk factors: a 6-month randomised controlled-feeding trial. Gut 68, 1417–1429 (2019).
Google Scholar
-
Vangay, P. et al. US immigration westernizes the human gut microbiome. Cell 175, 962–972.e10 (2018).
Google Scholar
-
Gutierrez Lopez, D. E., Lashinger, L. M., Weinstock, G. M. & Bray, M. S. Circadian rhythms and the gut microbiome synchronize the host’s metabolic response to diet. Cell Metab. 33, 873–887 (2021).
Google Scholar
-
Lotti, S., Dinu, M., Colombini, B., Amedei, A. & Sofi, F. Circadian rhythms, gut microbiota, and diet: possible implications for health. Nutr. Metab. Cardiovasc. Dis. 33, 1490–1500 (2023).
Google Scholar
-
Ratiner, K., Shapiro, H., Goldenberg, K. & Elinav, E. Time-limited diets and the gut microbiota in cardiometabolic disease. J. Diabetes 14, 377–393 (2022).
Google Scholar
-
Bermingham, K. M. et al. Exploring the relationship between social jetlag with gut microbial composition, diet and cardiometabolic health, in the ZOE PREDICT 1 cohort. Eur. J. Nutr. 62, 3135–3147 (2023).
Google Scholar
-
Zhernakova, A. et al. Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science 352, 565–569 (2016).
Google Scholar
-
Falony, G. et al. Population-level analysis of gut microbiome variation. Science 352, 560–564 (2016).
Google Scholar
-
Frankenfeld, C. L. et al. The gut microbiome is associated with circulating dietary biomarkers of fruit and vegetable intake in a multiethnic cohort. J. Acad. Nutr. Diet. 122, 78–98 (2022).
Google Scholar
-
Manor, O. et al. Health and disease markers correlate with gut microbiome composition across thousands of people. Nat. Commun. 11, 5206 (2020).
Google Scholar
-
Partula, V. et al. Associations between usual diet and gut microbiota composition: results from the Milieu Intérieur cross-sectional study. Am. J. Clin. Nutr. 109, 1472–1483 (2019).
Google Scholar
-
Maukonen, M. et al. Associations of plant-based foods, red and processed meat, and dairy with gut microbiome in Finnish adults. Eur. J. Nutr. 63, 2247–2260 (2024).
Google Scholar
-
Koponen, K. K. et al. Associations of healthy food choices with gut microbiota profiles. Am. J. Clin. Nutr. 114, 605–616 (2021).
Google Scholar
-
Yu, D. et al. Long-term diet quality is associated with gut microbiome diversity and composition among urban Chinese adults. Am. J. Clin. Nutr. 113, 684–694 (2021).
Google Scholar
-
Borrello, K. et al. Dietary intake mediates ethnic differences in gut microbial composition. Nutrients 14, 660 (2022).
Google Scholar
-
Latorre-Pérez, A. et al. The Spanish gut microbiome reveals links between microorganisms and Mediterranean diet. Sci. Rep. 11, 21602 (2021).
Google Scholar
-
Houttu, V. et al. Physical activity and dietary composition relate to differences in gut microbial patterns in a multi-ethnic cohort – the HELIUS study. Metabolites 11, 858 (2021).
Google Scholar
-
Le Roy, C. I. et al. Yoghurt consumption is associated with changes in the composition of the human gut microbiome and metabolome. BMC Microbiol. 22, 39 (2022).
Google Scholar
-
Jiang, Z. et al. Dietary fruit and vegetable intake, gut microbiota, and type 2 diabetes: results from two large human cohort studies. BMC Med. 18, 371 (2020).
Google Scholar
-
Aasmets, O., Krigul, K. L., Lüll, K., Metspalu, A. & Org, E. Gut metagenome associations with extensive digital health data in a volunteer-based Estonian microbiome cohort. Nat. Commun. 13, 869 (2022).
Google Scholar
-
Cotillard, A. et al. Dietary intervention impact on gut microbial gene richness. Nature 500, 585–588 (2013).
Google Scholar
-
Miller, V. et al. Global dietary quality in 185 countries from 1990 to 2018 show wide differences by nation, age, education, and urbanicity. Nat. Food 3, 694–702 (2022).
Google Scholar
-
Jardon, K. M., Canfora, E. E., Goossens, G. H. & Blaak, E. E. Dietary macronutrients and the gut microbiome: a precision nutrition approach to improve cardiometabolic health. Gut 71, 1214–1226 (2022).
Google Scholar
-
Arnone, D. et al. Sugars and gastrointestinal health. Clin. Gastroenterol. Hepatol. 20, 1912–1924.e7 (2022).
Google Scholar
-
Fernández-Bañares, F. Carbohydrate maldigestion and intolerance. Nutrients 14, 1923 (2022).
Google Scholar
-
Blachier, F. et al. High-protein diets for weight management: interactions with the intestinal microbiota and consequences for gut health. A position paper by the My New Gut study group. Clin. Nutr. 38, 1012–1022 (2019).
Google Scholar
-
Bartlett, A. & Kleiner, M. Dietary protein and the intestinal microbiota: an understudied relationship. iScience 25, 105313 (2022).
Google Scholar
-
Fassarella, M. et al. Gut microbiome stability and resilience: elucidating the response to perturbations in order to modulate gut health. Gut 70, 595–605 (2021).
Google Scholar
-
Li, D. et al. Gut microbiota-derived metabolite trimethylamine-N-oxide and multiple health outcomes: an umbrella review and updated meta-analysis. Am. J. Clin. Nutr. 116, 230–243 (2022).
Google Scholar
-
Wolters, M. et al. Dietary fat, the gut microbiota, and metabolic health – a systematic review conducted within the MyNewGut project. Clin. Nutr. 38, 2504–2520 (2019).
Google Scholar
-
Lemons, J. M. S. & Liu, L. Chewing the fat with microbes: lipid crosstalk in the gut. Nutrients 14, 573 (2022).
Google Scholar
-
Natividad, J. M. et al. Bilophila wadsworthia aggravates high fat diet induced metabolic dysfunctions in mice. Nat. Commun. 9, 2802 (2018).
Google Scholar
-
Collins, S. L., Stine, J. G., Bisanz, J. E., Okafor, C. D. & Patterson, A. D. Bile acids and the gut microbiota: metabolic interactions and impacts on disease. Nat. Rev. Microbiol. 21, 236–247 (2023).
Google Scholar
-
Pham, V. T., Dold, S., Rehman, A., Bird, J. K. & Steinert, R. E. Vitamins, the gut microbiome and gastrointestinal health in humans. Nutr. Res. 95, 35–53 (2021).
Google Scholar
-
Wilck, N. et al. Salt-responsive gut commensal modulates TH17 axis and disease. Nature 551, 585–589 (2017).
Google Scholar
-
Musiol, S. et al. The impact of high-salt diet on asthma in humans and mice: effect on specific T-cell signatures and microbiome. Allergy 79, 1844–1857 (2024).
Google Scholar
-
Spiga, L. et al. Iron acquisition by a commensal bacterium modifies host nutritional immunity during Salmonella infection. Cell Host Microbe 31, 1639–1654.e10 (2023).
Google Scholar
-
Martin, O. C. B. et al. Haem iron reshapes colonic luminal environment: impact on mucosal homeostasis and microbiome through aldehyde formation. Microbiome 7, 72 (2019).
Google Scholar
-
Mervant, L. et al. Urinary metabolome analysis reveals potential microbiota alteration and electrophilic burden induced by high red meat diet: results from the French NutriNet-Santé cohort and an in vivo intervention study in rats. Mol. Nutr. Food Res. 67, e2200432 (2023).
Google Scholar
-
Rowland, I. et al. Gut microbiota functions: metabolism of nutrients and other food components. Eur. J. Nutr. 57, 1–24 (2018).
Google Scholar
-
Pereira, Q. C. et al. Polyphenolic compounds: orchestrating intestinal microbiota harmony during aging. Nutrients 16, 1066 (2024).
Google Scholar
-
Meijnikman, A. S. et al. Microbiome-derived ethanol in nonalcoholic fatty liver disease. Nat. Med. 28, 2100–2106 (2022).
Google Scholar
-
Srour, B. et al. Ultra-processed foods and human health: from epidemiological evidence to mechanistic insights. Lancet Gastroenterol. Hepatol. 7, 1128–1140 (2022).
Google Scholar
-
Shalon, D. et al. Profiling the human intestinal environment under physiological conditions. Nature 617, 581–591 (2023).
Google Scholar
-
Quinn, R. A. et al. Global chemical effects of the microbiome include new bile-acid conjugations. Nature 579, 123–129 (2020).
Google Scholar
-
Di Vincenzo, F., Del Gaudio, A., Petito, V., Lopetuso, L. R. & Scaldaferri, F. Gut microbiota, intestinal permeability, and systemic inflammation: a narrative review. Intern. Emerg. Med. 19, 275–293 (2024).
Google Scholar
-
Chae, Y.-R., Lee, Y. R., Kim, Y.-S. & Park, H.-Y. Diet-induced gut dysbiosis and leaky gut syndrome. J. Microbiol. Biotechnol. 34, 747–756 (2024).
Google Scholar
-
Ito, H. et al. Soluble fiber viscosity affects both goblet cell number and small intestine mucin secretion in rats. J. Nutr. 139, 1640–1647 (2009).
Google Scholar
-
Desai, M. S. et al. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell 167, 1339–1353.e21 (2016).
Google Scholar
-
Belzer, C. Nutritional strategies for mucosal health: the interplay between microbes and mucin glycans. Trends Microbiol. 30, 13–21 (2022).
Google Scholar
-
Mukherjee, S. & Hooper, L. V. Antimicrobial defense of the intestine. Immunity 42, 28–39 (2015).
Google Scholar
-
Keir, M., Yi, T., Lu, T. & Ghilardi, N. The role of IL-22 in intestinal health and disease. J. Exp. Med. 217, e20192195 (2020).
Google Scholar
-
Feng, Z., Sun, R., Cong, Y. & Liu, Z. Critical roles of G protein-coupled receptors in regulating intestinal homeostasis and inflammatory bowel disease. Mucosal Immunol. 15, 819–828 (2022).
Google Scholar
-
Stockinger, B., Shah, K. & Wincent, E. AHR in the intestinal microenvironment: safeguarding barrier function. Nat. Rev. Gastroenterol. Hepatol. 18, 559–570 (2021).
Google Scholar
-
Kahalehili, H. M. et al. Dietary indole-3-carbinol activates AhR in the gut, alters Th17-microbe interactions, and exacerbates insulitis in NOD mice. Front. Immunol. 11, 606441 (2020).
Google Scholar
-
Song, Y. et al. Molecular and structural basis of interactions of vitamin D3 hydroxyderivatives with aryl hydrocarbon receptor (AhR): an integrated experimental and computational study. Int. J. Biol. Macromol. 209, 1111–1123 (2022).
Google Scholar
-
Bora, S. A., Kennett, M. J., Smith, P. B., Patterson, A. D. & Cantorna, M. T. The gut microbiota regulates endocrine vitamin D metabolism through fibroblast growth factor 23. Front. Immunol. 9, 408 (2018).
Google Scholar
-
Aoki, R., Aoki-Yoshida, A., Suzuki, C. & Takayama, Y. Indole-3-pyruvic acid, an aryl hydrocarbon receptor activator, suppresses experimental colitis in mice. J. Immunol. 201, 3683–3693 (2018).
Google Scholar
-
Woo, V. et al. Commensal segmented filamentous bacteria-derived retinoic acid primes host defense to intestinal infection. Cell Host Microbe 29, 1744–1756.e5 (2021).
Google Scholar
-
Han, X. et al. Dietary regulation of the SIgA–gut microbiota interaction. Crit. Rev. Food Sci. Nutr. 63, 6379–6392 (2023).
Google Scholar
-
Shin, S. Y. et al. Compositional changes in the gut microbiota of responders and non-responders to probiotic treatment among patients with diarrhea-predominant irritable bowel syndrome: a post hoc analysis of a randomized clinical trial. J. Neurogastroenterol. Motil. 28, 642–654 (2022).
Google Scholar
-
Yu, M. et al. Aryl hydrocarbon receptor activation modulates intestinal epithelial barrier function by maintaining tight junction integrity. Int. J. Biol. Sci. 14, 69–77 (2018).
Google Scholar
-
Feng, W. et al. Sodium butyrate attenuates diarrhea in weaned piglets and promotes tight junction protein expression in colon in a GPR109A-dependent manner. Cell. Physiol. Biochem. 47, 1617–1629 (2018).
Google Scholar
-
Li, X., Yao, Z., Qian, J., Li, H. & Li, H. Lactate protects intestinal epithelial barrier function from dextran sulfate sodium-induced damage by GPR81 signaling. Nutrients 16, 582 (2024).
Google Scholar
-
Venkatesh, M. et al. Symbiotic bacterial metabolites regulate gastrointestinal barrier function via the xenobiotic sensor PXR and toll-like receptor 4. Immunity 41, 296–310 (2014).
Google Scholar
-
He, C. et al. Vitamin A inhibits the action of LPS on the intestinal epithelial barrier function and tight junction proteins. Food Funct. 10, 1235–1242 (2019).
Google Scholar
-
Cho, Y.-E. et al. Fructose promotes leaky gut, endotoxemia, and liver fibrosis through ethanol-inducible cytochrome P450-2E1-mediated oxidative and nitrative stress. Hepatology 73, 2180–2195 (2021).
Google Scholar
-
Rohr, M. W., Narasimhulu, C. A., Rudeski-Rohr, T. A. & Parthasarathy, S. Negative effects of a high-fat diet on intestinal permeability: a review. Adv. Nutr. 11, 77–91 (2020).
Google Scholar
-
Chassaing, B. et al. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature 519, 92–96 (2015).
Google Scholar
-
Siracusa, F. et al. Short-term dietary changes can result in mucosal and systemic immune depression. Nat. Immunol. 24, 1473–1486 (2023).
Google Scholar
-
Tan, J. et al. Your regulatory T cells are what you eat: how diet and gut microbiota affect regulatory T cell development. Front. Nutr. 9, 878382 (2022).
Google Scholar
-
Bakdash, G., Vogelpoel, L. T. C., van Capel, T. M. M., Kapsenberg, M. L. & de Jong, E. C. Retinoic acid primes human dendritic cells to induce gut-homing, IL-10-producing regulatory T cells. Mucosal Immunol. 8, 265–278 (2015).
Google Scholar
-
Xie, L., Alam, M. J., Marques, F. Z. & Mackay, C. R. A major mechanism for immunomodulation: dietary fibres and acid metabolites. Semin. Immunol. 66, 101737 (2023).
Google Scholar
-
Kayama, H. & Takeda, K. Emerging roles of host and microbial bioactive lipids in inflammatory bowel diseases. Eur. J. Immunol. 53, e2249866 (2023).
Google Scholar
-
Urry, Z. et al. The role of 1α,25-dihydroxyvitamin D3 and cytokines in the promotion of distinct Foxp3+ and IL-10+ CD4+ T cells. Eur. J. Immunol. 42, 2697–2708 (2012).
Google Scholar
-
Quintana, F. J. et al. Control of Treg and TH17 cell differentiation by the aryl hydrocarbon receptor. Nature 453, 65–71 (2008).
Google Scholar
-
Kelly, B. & Pearce, E. L. Amino assets: how amino acids support immunity. Cell Metab. 32, 154–175 (2020).
Google Scholar
-
Bauché, D. & Marie, J. C. Transforming growth factor β: a master regulator of the gut microbiota and immune cell interactions. Clin. Transl. Immunol. 6, e136 (2017).
Google Scholar
-
Elias, K. M. et al. Retinoic acid inhibits Th17 polarization and enhances FoxP3 expression through a Stat-3/Stat-5 independent signaling pathway. Blood 111, 1013–1020 (2008).
Google Scholar
-
Paudel, S., Mishra, N. & Agarwal, R. Phytochemicals as immunomodulatory molecules in cancer therapeutics. Pharmaceuticals 16, 1652 (2023).
Google Scholar
-
Rodrigues, H. G., Takeo Sato, F., Curi, R. & Vinolo, M. A. R. Fatty acids as modulators of neutrophil recruitment, function and survival. Eur. J. Pharmacol. 785, 50–58 (2016).
Google Scholar
-
Schulthess, J. et al. The short chain fatty acid butyrate imprints an antimicrobial program in macrophages. Immunity 50, 432–445.e7 (2019).
Google Scholar
-
Nastasi, C. et al. Butyrate and propionate inhibit antigen-specific CD8+ T cell activation by suppressing IL-12 production by antigen-presenting cells. Sci. Rep. 7, 14516 (2017).
Google Scholar
-
Li, S., Bostick, J. W. & Zhou, L. Regulation of innate lymphoid cells by aryl hydrocarbon receptor. Front. Immunol. 8, 1909 (2017).
Google Scholar
-
Li, Y. et al. Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation. Cell 147, 629–640 (2011).
Google Scholar
-
Inagaki, T. et al. Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proc. Natl Acad. Sci. USA 103, 3920–3925 (2006).
Google Scholar
-
Gadaleta, R. M. et al. Farnesoid X receptor activation inhibits inflammation and preserves the intestinal barrier in inflammatory bowel disease. Gut 60, 463–472 (2011).
Google Scholar
-
Thibaut, M. M. & Bindels, L. B. Crosstalk between bile acid-activated receptors and microbiome in entero-hepatic inflammation. Trends Mol. Med. 28, 223–236 (2022).
Google Scholar
-
Zmora, N., Levy, M., Pevsner-Fishcer, M. & Elinav, E. Inflammasomes and intestinal inflammation. Mucosal Immunol. 10, 865–883 (2017).
Google Scholar
-
Swanson, K. V., Deng, M. & Ting, J. P.-Y. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 19, 477–489 (2019).
Google Scholar
-
Zheng, D. et al. Epithelial Nlrp10 inflammasome mediates protection against intestinal autoinflammation. Nat. Immunol. 24, 585–594 (2023).
Google Scholar
-
Macia, L. et al. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat. Commun. 6, 6734 (2015).
Google Scholar
-
Wen, H. et al. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat. Immunol. 12, 408–415 (2011).
Google Scholar
-
Yan, Y. et al. Omega-3 fatty acids prevent inflammation and metabolic disorder through inhibition of NLRP3 inflammasome activation. Immunity 38, 1154–1163 (2013).
Google Scholar
-
Schneider, E., O’Riordan, K. J., Clarke, G. & Cryan, J. F. Feeding gut microbes to nourish the brain: unravelling the diet-microbiota–gut–brain axis. Nat. Metab. 6, 1454–1478 (2024).
Google Scholar
-
Knox, E. G. et al. Microbial-derived metabolites induce actin cytoskeletal rearrangement and protect blood–brain barrier function. iScience 25, 105648 (2022).
Google Scholar
-
O’Riordan, K. J. et al. Short chain fatty acids: microbial metabolites for gut–brain axis signalling. Mol. Cell. Endocrinol. 546, 111572 (2022).
Google Scholar
-
Spichak, S. et al. Mining microbes for mental health: determining the role of microbial metabolic pathways in human brain health and disease. Neurosci. Biobehav. Rev. 125, 698–761 (2021).
Google Scholar
-
McGuinness, A. J. et al. A systematic review of gut microbiota composition in observational studies of major depressive disorder, bipolar disorder and schizophrenia. Mol. Psychiatry 27, 1920–1935 (2022).
Google Scholar
-
Nikolova, V. L. et al. Perturbations in gut microbiota composition in psychiatric disorders: a review and meta-analysis. JAMA Psychiatry 78, 1343–1354 (2021).
Google Scholar
-
Aslam, H. et al. Fiber intake and fiber intervention in depression and anxiety: a systematic review and meta-analysis of observational studies and randomized controlled trials. Nutr. Rev. 82, 1678–1695 (2024).
Google Scholar
-
Provensi, G. et al. Preventing adolescent stress-induced cognitive and microbiome changes by diet. Proc. Natl Acad. Sci. USA 116, 9644–9651 (2019).
Google Scholar
-
Kerman, B. E., Self, W. & Yassine, H. N. Can the gut microbiome inform the effects of omega-3 fatty acid supplementation trials on cognition? Curr. Opin. Clin. Nutr. Metab. Care 27, 116–124 (2024).
Google Scholar
-
Miyamoto, J. et al. Gut microbiota confers host resistance to obesity by metabolizing dietary polyunsaturated fatty acids. Nat. Commun. 10, 4007 (2019).
Google Scholar
-
Schverer, M. et al. Dietary phospholipids: role in cognitive processes across the lifespan. Neurosci. Biobehav. Rev. 111, 183–193 (2020).
Google Scholar
-
Ross, F. C. et al. Potential of dietary polyphenols for protection from age-related decline and neurodegeneration: a role for gut microbiota?. Nutr. Neurosci. 27, 1058–1076 (2024).
Google Scholar
-
Brickman, A. M. et al. Dietary flavanols restore hippocampal-dependent memory in older adults with lower diet quality and lower habitual flavanol consumption. Proc. Natl Acad. Sci. USA 120, e2216932120 (2023).
Google Scholar
-
Karbownik, M. S., Mokros, Ł. & Kowalczyk, E. Who benefits from fermented food consumption? A comparative analysis between psychiatrically Ill and psychiatrically healthy medical students. Int. J. Environ. Res. Public. Health 19, 3861 (2022).
Google Scholar
-
Balasubramanian, R., Schneider, E., Gunnigle, E., Cotter, P. D. & Cryan, J. F. Fermented foods: harnessing their potential to modulate the microbiota–gut–brain axis for mental health. Neurosci. Biobehav. Rev. 158, 105562 (2024).
Google Scholar
-
Caffrey, E. B., Sonnenburg, J. L. & Devkota, S. Our extended microbiome: the human-relevant metabolites and biology of fermented foods. Cell Metab. 36, 684–701 (2024).
Google Scholar
-
Berding, K. et al. Feed your microbes to deal with stress: a psychobiotic diet impacts microbial stability and perceived stress in a healthy adult population. Mol. Psychiatry 28, 601–610 (2023).
Google Scholar
-
Conn, K. A., Borsom, E. M. & Cope, E. K. Implications of microbe-derived γ-aminobutyric acid (GABA) in gut and brain barrier integrity and GABAergic signaling in Alzheimer’s disease. Gut Microbes 16, 2371950 (2024).
Google Scholar
-
Bermudez-Martin, P. et al. The microbial metabolite p-cresol induces autistic-like behaviors in mice by remodeling the gut microbiota. Microbiome 9, 157 (2021).
Google Scholar
-
Chambers, E. S. et al. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut 64, 1744–1754 (2015).
Google Scholar
-
Zhao, L. et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 359, 1151–1156 (2018).
Google Scholar
-
Pedersen, M. G. B. et al. Oral lactate slows gastric emptying and suppresses appetite in young males. Clin. Nutr. 41, 517–525 (2022).
Google Scholar
-
Gribble, F. M. & Reimann, F. Enteroendocrine cells: chemosensors in the intestinal epithelium. Annu. Rev. Physiol. 78, 277–299 (2016).
Google Scholar
-
Engelstoft, M. S. et al. Seven transmembrane G protein-coupled receptor repertoire of gastric ghrelin cells. Mol. Metab. 2, 376–392 (2013).
Google Scholar
-
Fukumoto, S. et al. Short-chain fatty acids stimulate colonic transit via intraluminal 5-HT release in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 284, R1269–R1276 (2003).
Google Scholar
-
De Vadder, F. et al. Microbiota-generated metabolites promote metabolic benefits via gut–brain neural circuits. Cell 156, 84–96 (2014).
Google Scholar
-
Marin, E. et al. Human tolerogenic dendritic cells regulate immune responses through lactate synthesis. Cell Metab. 30, 1075–1090.e8 (2019).
Google Scholar
-
Luo, Y. et al. Effects of lactate in immunosuppression and inflammation: progress and prospects. Int. Rev. Immunol. 41, 19–29 (2022).
Google Scholar
-
Jocken, J. W. E. et al. Short-chain fatty acids differentially affect intracellular lipolysis in a human white adipocyte model. Front. Endocrinol. 8, 372 (2017).
Google Scholar
-
Canfora, E. E. et al. Colonic infusions of short-chain fatty acid mixtures promote energy metabolism in overweight/obese men: a randomized crossover trial. Sci. Rep. 7, 2360 (2017).
Google Scholar
-
Hu, J. et al. The roles of GRP81 as a metabolic sensor and inflammatory mediator. J. Cell. Physiol. 235, 8938–8950 (2020).
Google Scholar
-
Reddy, A. et al. Monocarboxylate transporters facilitate succinate uptake into brown adipocytes. Nat. Metab. 6, 567–577 (2024).
Google Scholar
-
Kimura, I. et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat. Commun. 4, 1829 (2013).
Google Scholar
-
Villanueva-Carmona, T. et al. SUCNR1 signaling in adipocytes controls energy metabolism by modulating circadian clock and leptin expression. Cell Metab. 35, 601–619.e10 (2023).
Google Scholar
-
Müller, M. et al. Circulating but not faecal short-chain fatty acids are related to insulin sensitivity, lipolysis and GLP-1 concentrations in humans. Sci. Rep. 9, 12515 (2019).
Google Scholar
-
Frost, G. et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat. Commun. 5, 3611 (2014).
Google Scholar
-
Perry, R. J. et al. Acetate mediates a microbiome–brain–β-cell axis to promote metabolic syndrome. Nature 534, 213–217 (2016).
Google Scholar
-
Wang, K. et al. Parabacteroides distasonis alleviates obesity and metabolic dysfunctions via production of succinate and secondary bile acids. Cell Rep. 26, 222–235.e5 (2019).
Google Scholar
-
Osuna-Prieto, F. J. et al. Elevated plasma succinate levels are linked to higher cardiovascular disease risk factors in young adults. Cardiovasc. Diabetol. 20, 151 (2021).
Google Scholar
-
Serena, C. et al. Elevated circulating levels of succinate in human obesity are linked to specific gut microbiota. ISME J. 12, 1642–1657 (2018).
Google Scholar
-
Ding, Y. et al. Oral supplementation of gut microbial metabolite indole-3-acetate alleviates diet-induced steatosis and inflammation in mice. eLife 12, RP87458 (2024).
Google Scholar
-
Min, B. H. et al. Gut microbiota-derived indole compounds attenuate metabolic dysfunction-associated steatotic liver disease by improving fat metabolism and inflammation. Gut Microbes 16, 2307568 (2024).
Google Scholar
-
Wang, Z. et al. Gut microbiota and blood metabolites related to fiber intake and type 2 diabetes. Circ. Res. 134, 842–854 (2024).
Google Scholar
-
Dodd, D. et al. A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites. Nature 551, 648–652 (2017).
Google Scholar
-
Lu, C. et al. Indoxyl sulfate in atherosclerosis. Toxicol. Lett. 383, 204–212 (2023).
Google Scholar
-
Zhu, Y. et al. Two distinct gut microbial pathways contribute to meta-organismal production of phenylacetylglutamine with links to cardiovascular disease. Cell Host Microbe 31, 18–32.e9 (2023).
Google Scholar
-
Romano, K. A. et al. Gut microbiota-generated phenylacetylglutamine and heart failure. Circ. Heart Fail. 16, e009972 (2023).
Google Scholar
-
Han, H. et al. p-Cresyl sulfate aggravates cardiac dysfunction associated with chronic kidney disease by enhancing apoptosis of cardiomyocytes. J. Am. Heart Assoc. 4, e001852 (2015).
Google Scholar
-
Kok, C. R., Rose, D. & Hutkins, R. Predicting personalized responses to dietary fiber interventions: opportunities for modulation of the gut microbiome to improve health. Annu. Rev. Food Sci. Technol. 14, 157–182 (2023).
Google Scholar
-
Pedersen, H. K. et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature 535, 376–381 (2016).
Google Scholar
-
Wang, T. J. et al. Metabolite profiles and the risk of developing diabetes. Nat. Med. 17, 448–453 (2011).
Google Scholar
-
Fromentin, S. et al. Microbiome and metabolome features of the cardiometabolic disease spectrum. Nat. Med. 28, 303–314 (2022).
Google Scholar
-
Li, T.-T. et al. Microbiota metabolism of intestinal amino acids impacts host nutrient homeostasis and physiology. Cell Host Microbe 32, 661–675.e10 (2024).
Google Scholar
-
Choi, B. H., Hyun, S. & Koo, S.-H. The role of BCAA metabolism in metabolic health and disease. Exp. Mol. Med. 56, 1552–1559 (2024).
Google Scholar
-
Rigamonti, A. E. et al. The appetite-suppressant and GLP-1-stimulating effects of whey proteins in obese subjects are associated with increased circulating levels of specific amino acids. Nutrients 12, 775 (2020).
Google Scholar
-
Magkos, F. The role of dietary protein in obesity. Rev. Endocr. Metab. Disord. 21, 329–340 (2020).
Google Scholar
-
Kenny, D. J. et al. Cholesterol metabolism by uncultured human gut bacteria influences host cholesterol level. Cell Host Microbe 28, 245–257.e6 (2020).
Google Scholar
-
Romaní-Pérez, M. et al. Holdemanella biformis improves glucose tolerance and regulates GLP-1 signaling in obese mice. FASEB J. 35, e21734 (2021).
Google Scholar
-
Takeuchi, T. et al. Fatty acid overproduction by gut commensal microbiota exacerbates obesity. Cell Metab. 35, 361–375.e9 (2023).
Google Scholar
-
Wei, W. et al. Parabacteroides distasonis uses dietary inulin to suppress NASH via its metabolite pentadecanoic acid. Nat. Microbiol. 8, 1534–1548 (2023).
Google Scholar
-
Johnson, E. L. et al. Sphingolipids produced by gut bacteria enter host metabolic pathways impacting ceramide levels. Nat. Commun. 11, 2471 (2020).
Google Scholar
-
Liu, C. et al. Gut commensal Christensenella minuta modulates host metabolism via acylated secondary bile acids. Nat. Microbiol. 9, 434–450 (2024).
Google Scholar
-
Kuhre, R. E. et al. Bile acids are important direct and indirect regulators of the secretion of appetite- and metabolism-regulating hormones from the gut and pancreas. Mol. Metab. 11, 84–95 (2018).
Google Scholar
-
Zheng, X. et al. Hyocholic acid species improve glucose homeostasis through a distinct TGR5 and FXR signaling mechanism. Cell Metab. 33, 791–803.e7 (2021).
Google Scholar
-
Browning, M. G., Pessoa, B. M., Khoraki, J. & Campos, G. M. Changes in bile acid metabolism, transport, and signaling as central drivers for metabolic improvements after bariatric surgery. Curr. Obes. Rep. 8, 175–184 (2019).
Google Scholar
-
Makki, K. et al. 6α-hydroxylated bile acids mediate TGR5 signalling to improve glucose metabolism upon dietary fiber supplementation in mice. Gut 72, 314–324 (2023).
Google Scholar
-
Hang, S. et al. Bile acid metabolites control TH17 and Treg cell differentiation. Nature 576, 143–148 (2019).
Google Scholar
-
Wahlström, A. et al. Production of deoxycholic acid by low-abundant microbial species is associated with impaired glucose metabolism. Nat. Commun. 15, 4276 (2024).
Google Scholar
-
Guzior, D. V. et al. Bile salt hydrolase acyltransferase activity expands bile acid diversity. Nature 626, 852–858 (2024).
Google Scholar
-
Wu, H. et al. The gut microbiota in prediabetes and diabetes: a population-based cross-sectional study. Cell Metab. 32, 379–390.e3 (2020).
Google Scholar
-
Belda, E. et al. Impairment of gut microbial biotin metabolism and host biotin status in severe obesity: effect of biotin and prebiotic supplementation on improved metabolism. Gut 71, 2463–2480 (2022).
Google Scholar
-
Sun, Y. et al. Parabacteroides distasonis ameliorates insulin resistance via activation of intestinal GPR109a. Nat. Commun. 14, 7740 (2023).
Google Scholar
-
Man, A. W. C., Zhou, Y., Xia, N. & Li, H. Involvement of gut microbiota, microbial metabolites and interaction with polyphenol in host immunometabolism. Nutrients 12, 3054 (2020).
Google Scholar
-
Yoon, H. S. et al. Akkermansia muciniphila secretes a glucagon-like peptide-1-inducing protein that improves glucose homeostasis and ameliorates metabolic disease in mice. Nat. Microbiol. 6, 563–573 (2021).
Google Scholar
-
Grasset, E. et al. A specific gut microbiota dysbiosis of type 2 diabetic mice induces GLP-1 resistance through an enteric NO-dependent and gut–brain axis mechanism. Cell Metab. 25, 1075–1090.e5 (2017).
Google Scholar
-
Liang, C. et al. Ligilactobacillus salivarius LCK11 prevents obesity by promoting PYY secretion to inhibit appetite and regulating gut microbiota in C57BL/6J mice. Mol. Nutr. Food Res. 65, e2100136 (2021).
Google Scholar
-
Food and Agriculture Organization. Dietary guidelines. FAO www.fao.org/nutrition/education/food-dietary-guidelines/home/en/ (2025).
-
Hercberg, S., Touvier, M., Salas-Salvado, J. & Group of European scientists supporting the implementation of Nutri-Score in Europe. The nutri-score nutrition label. Int. J. Vitam. Nutr. Res. 92, 147–157 (2022).
Google Scholar
-
Nutri-Score European Scientific Committee. Nutri-Score. Santé publique, France www.santepubliquefrance.fr/en/nutri-score (2025).
-
Reyes, M. et al. Development of the Chilean front-of-package food warning label. BMC Public. Health 19, 906 (2019).
Google Scholar
-
Srour, B. et al. Effect of a new graphically modified Nutri-Score on the objective understanding of foods’ nutrient profile and ultraprocessing: a randomised controlled trial. BMJ Nutr. Prev. Health 6, 108–118 (2023).
Google Scholar
-
Merten, C. et al. Editorial: exploring the need to include microbiomes into EFSA’s scientific assessments. EFSA J. 18, e18061 (2020).
Google Scholar
-
Garcia-Vello, P. et al. Preparing for future challenges in risk assessment in the European Union. Trends Biotechnol. 40, 1137–1140 (2022).
Google Scholar
-
EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA) Scientific opinion on dietary reference values for carbohydrates and dietary fibre. EFSA J. 8, 1462 (2010).
-
Hoge Gezonheidsraad. Voedingsaanbevelingen voor Belgie. HGR Nr. 9285. HGR www.health.belgium.be/sites/default/files/uploads/fields/fpshealth_theme_file/9285_voedingsaanbev_16122016_a5.pdf (2016).
-
Vorster, H. H., Badham, J. B. & Venter, C. S. An introduction to the revised food-based dietary guidelines for South Africa. S. Afr. J. Clin. Nutr. 26, S5–S12 (2013).
-
Dietary Guidelines Advisory Committee. Scientific report of the 2020 Dietary Guidelines Advisory Committee: advisory report to the Secretary of Agriculture and the Secretary of Health and Human Services. Dietary Guidelines for Americans www.dietaryguidelines.gov/2020-advisory-committee-report (2020).
-
McKeown, N. M., Fahey, G. C., Slavin, J. & van der Kamp, J.-W. Fibre intake for optimal health: how can healthcare professionals support people to reach dietary recommendations? BMJ 378, e054370 (2022).
Google Scholar
-
Ou, J. et al. Diet, microbiota, and microbial metabolites in colon cancer risk in rural Africans and African Americans. Am. J. Clin. Nutr. 98, 111–120 (2013).
Google Scholar
-
Zmora, N., Suez, J. & Elinav, E. You are what you eat: diet, health and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 16, 35–56 (2019).
Google Scholar
-
Appel, L. J. et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH collaborative research group. N. Engl. J. Med. 336, 1117–1124 (1997).
Google Scholar
-
Paeslack, N. et al. Microbiota-derived tryptophan metabolites in vascular inflammation and cardiovascular disease. Amino Acids 54, 1339–1356 (2022).
Google Scholar
-
Kaye, D. M. et al. Deficiency of prebiotic fiber and insufficient signaling through gut metabolite-sensing receptors leads to cardiovascular disease. Circulation 141, 1393–1403 (2020).
Google Scholar
-
Meslier, V. et al. Mediterranean diet intervention in overweight and obese subjects lowers plasma cholesterol and causes changes in the gut microbiome and metabolome independently of energy intake. Gut 69, 1258–1268 (2020).
Google Scholar
-
Shoer, S. et al. Impact of dietary interventions on pre-diabetic oral and gut microbiome, metabolites and cytokines. Nat. Commun. 14, 5384 (2023).
Google Scholar
-
Dinan, T. G. et al. Feeding melancholic microbes: MyNewGut recommendations on diet and mood. Clin. Nutr. 38, 1995–2001 (2019).
Google Scholar
-
Morris, M. C. et al. MIND diet slows cognitive decline with aging. Alzheimers Dement. 11, 1015–1022 (2015).
Google Scholar
-
Agarwal, P. et al. MIND diet associated with reduced incidence and delayed progression of parkinsonism in old age. J. Nutr. Health Aging 22, 1211–1215 (2018).
Google Scholar
-
Erny, D. et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 18, 965–977 (2015).
Google Scholar
-
MahmoudianDehkordi, S. et al. Altered bile acid profile associates with cognitive impairment in Alzheimer’s disease – an emerging role for gut microbiome. Alzheimers Dement. 15, 76–92 (2019).
Google Scholar
-
Valls-Pedret, C. et al. Polyphenol-rich foods in the Mediterranean diet are associated with better cognitive function in elderly subjects at high cardiovascular risk. J. Alzheimers Dis. 29, 773–782 (2012).
Google Scholar
-
Torabynasab, K. et al. Adherence to the MIND diet is inversely associated with odds and severity of anxiety disorders: a case-control study. BMC Psychiatry 23, 330 (2023).
Google Scholar
-
Prince, A. C. et al. Fermentable carbohydrate restriction (low FODMAP diet) in clinical practice improves functional gastrointestinal symptoms in patients with inflammatory bowel disease. Inflamm. Bowel Dis. 22, 1129–1136 (2016).
Google Scholar
-
Kamphuis, J. B. J. et al. Lactose and fructo-oligosaccharides increase visceral sensitivity in mice via glycation processes, increasing mast cell density in colonic mucosa. Gastroenterology 158, 652–663.e6 (2020).
Google Scholar
-
Wielgosz-Grochowska, J. P., Domanski, N. & Drywień, M. E. Efficacy of an irritable bowel syndrome diet in the treatment of small intestinal bacterial overgrowth: a narrative review. Nutrients 14, 3382 (2022).
Google Scholar
-
Garg, P., Garg, P. K. & Bhattacharya, K. Psyllium husk positively alters gut microbiota, decreases inflammation, and has bowel-regulatory action, paving the way for physiologic management of irritable bowel syndrome. Gastroenterology 166, 545–546 (2024).
Google Scholar
-
Sanz, Y. Microbiome and gluten. Ann. Nutr. Metab. 67, 28–41 (2015).
Google Scholar
-
Caminero, A. et al. Duodenal bacterial proteolytic activity determines sensitivity to dietary antigen through protease-activated receptor-2. Nat. Commun. 10, 1198 (2019).
Google Scholar
-
Matera, M. & Guandalini, S. How the microbiota may affect celiac disease and what we can do. Nutrients 16, 1882 (2024).
Google Scholar
-
Adolfsen, K. J. et al. Improvement of a synthetic live bacterial therapeutic for phenylketonuria with biosensor-enabled enzyme engineering. Nat. Commun. 12, 6215 (2021).
Google Scholar
-
Bassanini, G. et al. Phenylketonuria diet promotes shifts in Firmicutes populations. Front. Cell. Infect. Microbiol. 9, 101 (2019).
Google Scholar
-
Verduci, E. et al. Phenylketonuric diet negatively impacts on butyrate production. Nutr. Metab. Cardiovasc. Dis. 28, 385–392 (2018).
Google Scholar
-
Misselwitz, B., Butter, M., Verbeke, K. & Fox, M. R. Update on lactose malabsorption and intolerance: pathogenesis, diagnosis and clinical management. Gut 68, 2080–2091 (2019).
Google Scholar
-
Azcarate-Peril, M. A. et al. A double-blind, 377-subject randomized study identifies Ruminococcus, Coprococcus, Christensenella, and Collinsella as long-term potential key players in the modulation of the gut microbiome of lactose intolerant individuals by galacto-oligosaccharides. Gut Microbes 13, 1957536 (2021).
Google Scholar
-
Angima, G., Qu, Y., Park, S. H. & Dallas, D. C. Prebiotic strategies to manage lactose intolerance symptoms. Nutrients 16, 1002 (2024).
Google Scholar
-
Ang, Q. Y. et al. Ketogenic diets alter the gut microbiome resulting in decreased intestinal Th17 cells. Cell 181, 1263–1275.e16 (2020).
Google Scholar
-
Olson, C. A. et al. The gut microbiota mediates the anti-seizure effects of the ketogenic diet. Cell 173, 1728–1741.e13 (2018).
Google Scholar
-
Li, Q., Liang, J., Fu, N., Han, Y. & Qin, J. A ketogenic diet and the treatment of autism spectrum disorder. Front. Pediatr. 9, 650624 (2021).
Google Scholar
-
Muscogiuri, G. et al. The management of very low-calorie ketogenic diet in obesity outpatient clinic: a practical guide. J. Transl. Med. 17, 356 (2019).
Google Scholar
-
Fitzpatrick, J. A., Melton, S. L., Yao, C. K., Gibson, P. R. & Halmos, E. P. Dietary management of adults with IBD – the emerging role of dietary therapy. Nat. Rev. Gastroenterol. Hepatol. 19, 652–669 (2022).
Google Scholar
-
Levine, A. et al. Crohn’s disease exclusion diet plus partial enteral nutrition induces sustained remission in a randomized controlled trial. Gastroenterology 157, 440–450.e8 (2019).
Google Scholar
-
Armstrong, H., Mander, I., Zhang, Z., Armstrong, D. & Wine, E. Not all fibers are born equal; variable response to dietary fiber subtypes in IBD. Front. Pediatr. 8, 620189 (2020).
Google Scholar
-
Hume, M. P., Nicolucci, A. C. & Reimer, R. A. Prebiotic supplementation improves appetite control in children with overweight and obesity: a randomized controlled trial. Am. J. Clin. Nutr. 105, 790–799 (2017).
Google Scholar
-
Birkeland, E. et al. Prebiotic effect of inulin-type fructans on faecal microbiota and short-chain fatty acids in type 2 diabetes: a randomised controlled trial. Eur. J. Nutr. 59, 3325–3338 (2020).
Google Scholar
-
Limketkai, B. N. et al. Prebiotics for induction and maintenance of remission in inflammatory bowel disease: systematic review and meta-analysis. Inflamm. Bowel Dis. 31, 1220–1230 (2025).
Google Scholar
-
Talukdar, J. R. et al. The effects of inulin-type fructans on cardiovascular disease risk factors: systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 119, 496–510 (2024).
Google Scholar
-
Arifuzzaman, M. et al. Inulin fibre promotes microbiota-derived bile acids and type 2 inflammation. Nature 611, 578–584 (2022).
Google Scholar
-
Yang, J. et al. High soluble fiber promotes colorectal tumorigenesis through modulating gut microbiota and metabolites in mice. Gastroenterology 166, 323–337.e7 (2024).
Google Scholar
-
Siscovick, D. S. et al. Omega-3 polyunsaturated fatty acid (fish oil) supplementation and the prevention of clinical cardiovascular disease: a Science Advisory from the American Heart Association. Circulation 135, e867–e884 (2017).
Google Scholar
-
La Rosa, F. et al. The gut-brain axis in Alzheimer’s disease and omega-3. A critical overview of clinical trials. Nutrients 10, 1267 (2018).
Google Scholar
-
Okburan, G. & Kızıler, S. Human milk oligosaccharides as prebiotics. Pediatr. Neonatol. 64, 231–238 (2023).
Google Scholar
-
Sanders, M. E., Merenstein, D. J., Reid, G., Gibson, G. R. & Rastall, R. A. Probiotics and prebiotics in intestinal health and disease: from biology to the clinic. Nat. Rev. Gastroenterol. Hepatol. 16, 605–616 (2019).
Google Scholar
-
Suez, J., Zmora, N., Segal, E. & Elinav, E. The pros, cons, and many unknowns of probiotics. Nat. Med. 25, 716–729 (2019).
Google Scholar
-
Veiga, P., Suez, J., Derrien, M. & Elinav, E. Moving from probiotics to precision probiotics. Nat. Microbiol. 5, 878–880 (2020).
Google Scholar
-
Michaudel, C. et al. Rewiring the altered tryptophan metabolism as a novel therapeutic strategy in inflammatory bowel diseases. Gut 72, 1296–1307 (2023).
Google Scholar
-
Milani, G. P. et al. A systematic review and meta-analysis on nutritional and dietary interventions for the treatment of acute respiratory infection in pediatric patients: An EAACI taskforce. Allergy 79, 1687–1707 (2024).
Google Scholar
-
Manson, J. E. et al. The Women’s Health Initiative randomized trials and clinical practice: a review. JAMA 331, 1748–1760 (2024).
Google Scholar
-
Dehghan, M. et al. Associations of fats and carbohydrate intake with cardiovascular disease and mortality in 18 countries from five continents (PURE): a prospective cohort study. Lancet 390, 2050–2062 (2017).
Google Scholar
-
Zeisel, S. H. Precision (personalized) nutrition: understanding metabolic heterogeneity. Annu. Rev. Food Sci. Technol. 11, 71–92 (2020).
Google Scholar
-
Lee, B. Y. et al. Research gaps and opportunities in precision nutrition: an NIH workshop report. Am. J. Clin. Nutr. 116, 1877–1900 (2022).
Google Scholar
-
Hjorth, M. F. et al. Pre-treatment microbial Prevotella-to-Bacteroides ratio, determines body fat loss success during a 6-month randomized controlled diet intervention. Int. J. Obes. 42, 580–583 (2018).
Google Scholar
-
Christensen, L. et al. Prevotella abundance predicts weight loss success in healthy, overweight adults consuming a whole-grain diet ad libitum: a post hoc analysis of a 6-wk randomized controlled trial. J. Nutr. 149, 2174–2181 (2019).
Google Scholar
-
Hur, H. J. et al. Beneficial effects of a low-glycemic diet on serum metabolites and gut microbiota in obese women with Prevotella and Bacteriodes enterotypes: a randomized clinical trial. Front. Nutr. 9, 861880 (2022).
Google Scholar
-
Dao, M. C. et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut 65, 426–436 (2016).
Google Scholar
-
Gargari, G. et al. Collinsella aerofaciens as a predictive marker of response to probiotic treatment in non-constipated irritable bowel syndrome. Gut Microbes 16, 2298246 (2024).
Google Scholar
-
Son, J., Jang, L.-G., Kim, B.-Y., Lee, S. & Park, H. The effect of athletes’ probiotic intake may depend on protein and dietary fiber intake. Nutrients 12, 2947 (2020).
Google Scholar
-
Wastyk, H. C. et al. Randomized controlled trial demonstrates response to a probiotic intervention for metabolic syndrome that may correspond to diet. Gut Microbes 15, 2178794 (2023).
Google Scholar
-
Zhang, C. et al. Ecological robustness of the gut microbiota in response to ingestion of transient food-borne microbes. ISME J. 10, 2235–2245 (2016).
Google Scholar
-
Zmora, N. et al. Personalized gut mucosal colonization resistance to empiric probiotics is associated with unique host and microbiome features. Cell 174, 1388–1405.e21 (2018).
Google Scholar
-
Nguyen, N. K. et al. Gut microbiota modulation with long-chain corn bran arabinoxylan in adults with overweight and obesity is linked to an individualized temporal increase in fecal propionate. Microbiome 8, 118 (2020).
Google Scholar
-
Rodriguez, J. et al. Discovery of the gut microbial signature driving the efficacy of prebiotic intervention in obese patients. Gut 69, 1975–1987 (2020).
Google Scholar
-
Leshem, A., Segal, E. & Elinav, E. The gut microbiome and individual-specific responses to diet. mSystems 5, e00665-20 (2020).
Google Scholar
-
Wu, W.-K. et al. Characterization of TMAO productivity from carnitine challenge facilitates personalized nutrition and microbiome signatures discovery. Microbiome 8, 162 (2020).
Google Scholar
-
Cohen, Y., Valdés-Mas, R. & Elinav, E. The role of artificial intelligence in deciphering diet-disease relationships: case studies. Annu. Rev. Nutr. 43, 225–250 (2023).
Google Scholar
-
Zeevi, D. et al. Personalized nutrition by prediction of glycemic responses. Cell 163, 1079–1094 (2015).
Google Scholar
-
Rein, M. et al. Effects of personalized diets by prediction of glycemic responses on glycemic control and metabolic health in newly diagnosed T2DM: a randomized dietary intervention pilot trial. BMC Med. 20, 56 (2022).
Google Scholar
-
Ben-Yacov, O. et al. Personalized postprandial glucose response-targeting diet versus Mediterranean diet for glycemic control in prediabetes. Diabetes Care 44, 1980–1991 (2021).
Google Scholar
-
Berry, S. E. et al. Human postprandial responses to food and potential for precision nutrition. Nat. Med. 26, 964–973 (2020).
Google Scholar
-
Valdés-Mas, R. et al. Metagenome-informed metaproteomics of the human gut microbiome, host, and dietary exposome uncovers signatures of health and inflammatory bowel disease. Cell 188, 1062–1083.e36 (2025).
Google Scholar
Acknowledgements
The work of Y.S. is funded by the Spanish Ministry of Science, Innovation and Universities (grant PID2023-150693OB-I00), and a “Severo Ochoa” grant of the National Agency for Research (AEI)–Spanish Ministry of Science and Innovation (ref. CEX2021-001189-S). The work of P.V. is funded in part by a Metagenopolis grant (ANR-11-DPBS-0001) and the PEPR-SAMS Cohortes-Microbiomes Project (ANR-24-PESA-0005). J.F.C. is supported by Science Foundation Ireland (SFI/12/RC/2273_P2), Saks Kavanaugh Foundation and Swiss National Science Foundation (CRSII5_186346/NMS2068). E.E. is supported by the European Union Thrive and Nutriome Consortiums and is a partner, Novo Nordisk Foundation Microbiome Health Initiative (MHI). R.L. is grateful to the Azrieli Foundation for an Azrieli Fellowship.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the preparing the manuscript.
Corresponding author
Ethics declarations
Competing interests
Y.S. is a co-author of probiotic patents, serves as a scientific adviser for Arla Foods and has been awarded by IFF. P.V. serves as a scientific consultant and adviser for Arla Foods and Newroad Innovations Ltd. J.F.C. is the co-author of probiotic patents and has received research funding from IFF, Nutricia and Kerry Foods, and has been an invited speaker at meetings organized by Bromotech and Nestlé. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Gastroenterology & Hepatology thanks Marie Arrieta and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Cite this article
Sanz, Y., Cryan, J.F., Deschasaux-Tanguy, M. et al. The gut microbiome connects nutrition and human health.
Nat Rev Gastroenterol Hepatol (2025). https://doi.org/10.1038/s41575-025-01077-5
-
Accepted: 02 May 2025
-
Published: 04 June 2025
-
DOI: https://doi.org/10.1038/s41575-025-01077-5