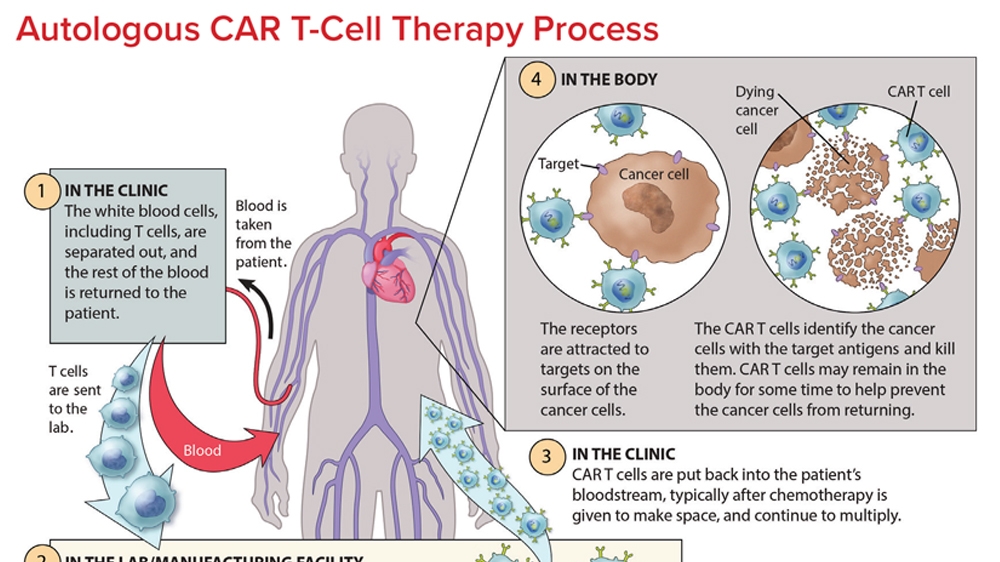
CAR T-cell therapy is an innovative approach to cancer treatment that has shown promising benefits, particularly in treating certain types of cancer. This groundbreaking therapy involves modifying a patient’s own immune cells to attack cancer cells, potentially leading to long-term remission. However, like all medical treatments, CAR T-cell therapy is not without its risks and challenges. These include severe side effects and complications, such as cytokine release syndrome and neurotoxicity. Therefore, the balance between the benefits and risks of CAR T-cell therapy remains a critical consideration for healthcare professionals and patients.
Secondary T-Cell Malignancies and CAR T-Cell Therapy
Recent reports have raised concerns about the possibility of patients developing secondary T-cell malignancies following treatment with CAR T-cell immunotherapy. This has prompted the US Food and Drug Administration (FDA) to add a class-wide boxed warning to the labeling of these therapies. Despite these concerns, most specialists agree that the overall benefits of CAR T-cell therapy continue to outweigh the risks.
The FDA has received 28 reports of T-cell malignancies following CAR T-cell treatment, with three cases showing evidence of likely CAR T-cell involvement. These cases involved five of the six FDA-approved CAR-T products. However, the numbers are too low to suggest an association with any particular product. Moreover, even if all the reported cases are assumed to be related to CAR-T treatment, they represent a very small proportion of the more than 27,000 doses of the six CAR-T therapies approved in the United States.
Lifelong Monitoring and Continued Research
The FDA is urging clinicians to monitor patients treated with CAR-T therapy for lifelong for new malignancies. For instance, a case of T-cell lymphoma occurred in a patient with non-Hodgkin B-cell lymphoma three months after an anti-CD19 CAR T-cell treatment. A subsequent analysis of 449 patients treated with CAR-T therapy showed that 16 patients (3.6%) developed a secondary primary malignancy.
Despite these risks, experts recommend cautious reassurance when discussing the issue with patients. They emphasize that the benefits of CAR T therapy far outweigh the risks in relapsed/refractory malignancies. Therefore, lifelong monitoring of patients and continued research efforts are crucial to further assess risk and optimize patient selection criteria.
Looking Ahead
CAR T-cell therapy is indeed a significant breakthrough in cancer treatment. It has effectively saved and extended the lives of adults and children with forms of leukemia, lymphoma, and multiple myeloma, achieving long-lasting remission. The risk of secondary T-cell lymphoma appears to be similar to that of patients treated with other cancer therapies. Therefore, despite the risks, the FDA and stakeholders believe that the benefits of CAR-T treatment outweigh the risks.
While it is crucial to continue exploring ways to minimize the risks associated with CAR T-cell therapy, it is equally important to recognize the revolutionary potential of this treatment. As research and clinical trials continue, we can look forward to more refined approaches to CAR T-cell therapy that will further enhance its benefits and reduce its risks.