Abstract
Background Despite the beneficial effects of a plant-based diet on colorectal cancer (CRC), no study has yet investigated the relationship between a Planetary Health Diet Index (PHDI) and CRC in the Iranian population. Therefore, the present case-control study aimed to assess the relationship between this index and CRC. Methods The current research was conducted on 71 patients with CRC (case group) and 142 (control group) admitted to hospitals in Tehran, Iran. The PHDI (0-150 points) was calculated based on a semi-quantitative food frequency questionnaire. Conditional logistic regression was applied to evaluate the association between CRC and PHDI and its sub-scores. Results After adjusting for the role of potential confounders, lower odds of CRC were observed in the second tertile of the total ratio score (odds ratio (OR) = 0.334; 95% confidence interval (CI): 0.127–0.878, P = 0.026) and the last tertile of PHDI (OR = 0.407; 95% CI: 0.183–0.907, P = 0.028), total adequacy score (OR = 0.261; 95% CI: 0.110–0.622, P = 0.002), and total moderation score (OR = 0.380; 95% CI: 0.162–0.891, P = 0.026) in comparison to the first tertile of each index. Conclusions The current study’s findings indicated a reverse relationship between PHDI, total adequacy, moderation, and ratio scores with the CRC odds. However, it is suggested that more research be performed in this field in the future to confirm the results of this study.
Introduction
Colorectal cancer (CRC) is the second leading cause of cancer-related mortality and the third most diagnosed cancer among women and men worldwide1. This incidence is higher in low- and middle-income developing countries and is increasing rapidly2. Diet plays a significant role in CRC pathogenesis3. Based on previous studies, increasing the intake of plant-based foods (legumes, vegetables, and fruits) containing bioactive components such as polyphenols and fibers, as well as dairy and calcium, and decreasing the consumption of red and processed meat, fat, sugar, and alcohol are related to a reduction in the risk of CRC4,5,6,7. These bioactive components prevent the development of CRC through their anti-inflammatory, antioxidant, anticancer, and antiproliferative effects8,9.
In January 2019, the EAT-Lancet Commission introduced a comprehensive and healthy reference diet. This diet aims to realign global food systems, enhance environmental sustainability, and promote human health10. The diet primarily focuses on plant-based foods such as legumes, vegetables, fruits, nuts, whole grains, and unsaturated oils. It also includes low to moderate amounts of poultry and seafood while limiting the intake of starchy vegetables, refined grains, added sugar, processed meat, and red meat11. In 2021, the Planetary Health Diet Index (PHDI) was developed and validated, largely inspired by the recommendations of the EAT-Lancet diet (ELD)12. This index emphasizes greater consumption of whole grains, fruits, greens, and vegetables, and lower consumption of tubers, refined grains, eggs, fish, and meat12.
According to previous studies, plant-based diets are related to a reduced CRC risk13,14. Also, in a cohort study conducted by Ren et al. on 98,415 American adults, a significant association was observed between ELD and CRC risk15. Furthermore, in a study by Berthy et al., a significant association was found between ELD and the risk of overall cancer only among females16.
Despite the beneficial effects of a plant-based diet on CRC, no study has yet investigated the relationship between PHDI and CRC in the Iranian population. Therefore, the present case-control study aimed to assess the relationship between this index and CRC.
Methods
This case-control study was conducted in three general hospitals and 19 CRC surgery departments in the Cancer Department of Imam Khomeini Hospital in Tehran, Iran. In the case group, individuals aged 40 to 75 years without a history of adenomatous polyposis or a previous cancer diagnosis in other organs, and newly diagnosed with CRC through pathological evaluation, were included. This study was matched for age (5-year intervals) and sex. The study sample size was calculated based on Terry et al.‘s study, with α = 0.05, β = 0.20, and odds ratio (OR) = 0.4517.
The people in the control group admitted due to non-neoplastic and acute conditions were randomly selected from the same hospitals simultaneously. The most common causes of hospitalization included disc disorders, osteoarticular disorders, fractures, and sprains.
At the beginning of the research, 178 and 89 people were chosen as the control and case groups, respectively. Accordingly, 24 individuals (8 cases and 16 controls) were excluded from the study due to unwillingness and incomplete data, as well as 20 participants in the control group and 10 participants in the case group (30 people in total) were excluded due to total energy intake (out of the mean ± 3 standard deviations (SDs) ( 3764 kcal/d for men, and 3397 kcal/d for women)), and the incompleteness of the food questionnaire (> 40% of food items were not responded). Finally, 142 and 71 people remained in the control and case groups, respectively. More details of the present study have been previously published18,19,20.
Dietary evaluation
A valid semi-quantitative food frequency questionnaire (FFQ), with confirmed reliability and validity21, was used to investigated dietary habits relative to the previous year. Initially, a valid food album and some household measurements (such as teaspoons, tablespoons, cups, plates, etc.)22 were used to facilitate the subjects’ response to the type and portion size of food consumption. Participants were then asked to report the frequency of each food item (daily, weekly, monthly, and yearly). Subsequently, data from the FFQ were converted to intake per day, and food consumption was calculated in grams22. Finally, Nutritionist IV was used to compute nutrient and energy intake23.
Diet quality scores
For the calculation of the PHDI score, we used the reference diet of EAT-Lancet that was formulated by the Commission of EAT-Lancet10. PHDI contains all food groups of EAT-Lancet, encompassing components that can be scored based on consumption. Scores were calculated as a ratio of caloric intake. In this way, the numerator is the total sum of the foods classified by caloric value in the component, and the denominator is the total sum of the foods in the PHDI consisting of sixteen components classified into four items:
-
1.
Adequacy items: total vegetables, legumes, peanuts and nuts, whole grains, and fruits.
-
2.
Optimal items: tubers and potatoes, fish and seafood, eggs, vegetable oils, and dairy products.
-
3.
Ratio items: orange and red vegetables in total vegetables and dark green vegetables in total vegetables.
-
4.
Moderation items: added sugars, animal fat, red meat, chicken, and alternatives.
While the components of the moderation, optimality, and adequacy items are scored between 0 and 10 points, the maximum score for the components of the ratio items is 5 points. As a result, the total score can be 0-150. Willett et al.’s study provided further details on the rationale and definition of the ELD10, and more details on the cut points, scoring criteria, and development of the PHDI were provided by Cacau et al.’s study12.
Anthropometric and socio-demographic assessments
Trained interviewers collected information such as socio-demographic characteristics, CRC history, physical activity, and medication usage using a checklist. Anthropometric indices were determined. Weight and height were assessed with an accuracy of 0.1 kg and 0.1 cm, respectively. Body mass index (BMI) was computed using the following formula:
BMI = Weight (kg) / height (m) 2.
Also, to assess physical activity, the International Physical Activity Questionnaire (IPAQ) was used24. In the control group, the activities of the year before the interview were considered, and in the case group, the activities of the year before the diagnosis of CRC were considered.
Statistical analysis
To evaluate the normality of the data, the Kolmogorov-Smirnov test was used. Additionally, the Mann-Whitney U and independent samples T-tests were utilized for non-parametric and parametric variables, respectively. Continuous variables were presented as median (confidence interval (CI)) or mean (SD), and categorical variables as frequency (percentage). Furthermore, to evaluate the association between CRC and PHDI and subgroups in two crude and adjusted models, conditional logistic regression was applied. In the adjusted model, variables with a p-value < 0.25 were selected based on Table 1. Therefore, income (dollars), smoking status (never/former/current), family history of CRC in first- and second-degree relatives (no/yes), and taking ibuprofen (no/yes), aspirin (no/yes), and acetaminophen (no/yes) were included in the multivariable model. R software (version 3.0.2) was used to illustrate all the figures. Additionally, SPSS (version 26.0) was used to analyze the data. A p-value < 0.05 was considered statistically significant.
Ethics approval and consent to participate
This study was conducted in accordance with the ethical standards of the Declaration of Helsinki and was approved by the Medical Research and Ethics Committee of Shiraz University of Medical Sciences (IR.SUMS.SCHEANUT.REC.1401.011). Informed consent was obtained from all participants and their legal guardian(s).
Results
Table 1 indicates the participants’ characteristics of the current study. The mean age of the control and case group participants was 57.7 and 58.2, respectively. There were significant differences between the two groups in total adequacy score (p < 0.001), total optimum score (p = 0.005), total moderation score (p = 0.028), fiber intake (p < 0.001), family history of CRC in first- (p = 0.017) and second-degree relatives (p = 0.006), and taking aspirin (p = 0.016), and acetaminophen (p = 0.004).
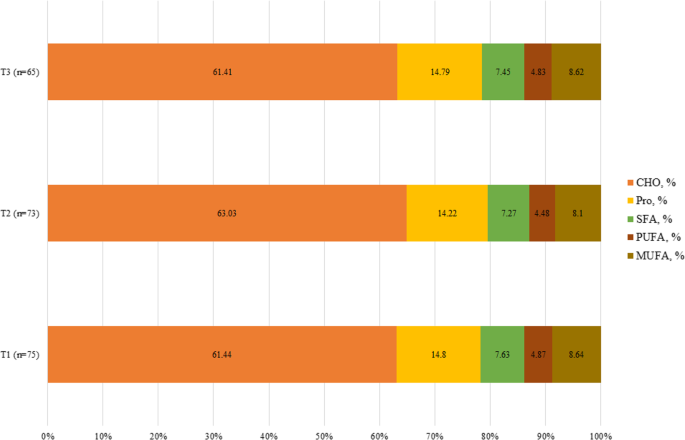
As presented in Fig. 1, macronutrient intake was significant only for protein (P = 0.042), but carbohydrate (P = 0.079), saturated fatty acids (SFAs) (P = 0.444), monounsaturated fatty acids (MUFA) (P = 0.063), and polyunsaturated fatty acids (PUFA) (P = 0.092) were not significant. Food item consumption in the total population and tertiles of PHDI score is shown in Table 2. According to Table 2, consumption of whole grains (P < 0.001), eggs (P = 0.001), chicken (P = 0.003), legumes (P = 0.015), animal fats (P < 0.001), and added sugars (P < 0.001) based on energy percent was different among the tertiles of PHDI.

The mean intake of macronutrients based on the tertiles of PHDI score.
The association between some baseline variables and CRC is shown in Table 3. In the univariate analysis, compared to individuals without a family history of CRC in first- and second-degree relatives, higher odds of CRC were observed in participants with a positive history (first-degree: OR = 5.068, 95% CI: 1.269–20.235, P = 0.022 – second-degree: OR = 13.015, 95% CI: 1.535-110.325, P = 0.019). Furthermore, we observed lower odds of CRC in participants taking acetaminophen compared to another group (OR = 0.243, 95% CI: 0.082–0.723, P = 0.011).
Table 4 represents the association between the PHDI and subgroups with CRC. In the crude model, the last tertile of PHDI and total adequacy score had lower odds of CRC compared to the first tertile (PHDI: OR = 0.392; 95% CI: 0.178–0.864, P = 0.020 – total adequacy score: OR = 0.350; 95% CI: 0.157–0.782, P = 0.010). Also, lower odds of CRC were seen in the second tertile of total ratio score in comparison to the first tertile (OR = 0.361; 95% CI: 0.138–0.946, P = 0.038).
In the second model, after adjusting for the role of potential confounders, lower odds of CRC were observed in the second tertile of the total ratio score (OR = 0.334; 95% CI: 0.127–0.878, P = 0.026), and the last tertile of PHDI (OR = 0.407; 95% CI: 0.183–0.907, P = 0.028), total adequacy score (OR = 0.261; 95% CI: 0.110–0.622, P = 0.002), and total moderation score (OR = 0.380; 95% CI: 0.162–0.891, P = 0.026), in comparison to the first tertile of each index.
Discussion
This case-control research was the first to examine the relationship between PHDI and CRC in an Iranian population. Based on our findings, a reverse relationship between PHDI, total adequacy, moderation, and ratio scores with CRC was observed.
PHDI is a plant-based index recommended by the EAT-Lancet Commission, which focuses on increasing the consumption of whole grains, fruits, vegetables, and greens and reducing the consumption of tubers, refined grains, meat, fish, and eggs to improve the health of the planet and the population10.
According to previous studies, a reverse association has been reported between the Mediterranean Diet (MD) Score, Healthy Eating Index, and the Dietary Approaches to Stop Hypertension (DASH) diet with CRC25,26. Consistent with the present study, a case-control study conducted in Canada indicated that a plant-based dietary pattern protects against CRC. In contrast, sugary and meat-based dietary patterns are related to a higher CRC risk27. Additionally, based on the results of a North American cohort, after a 7.5-year follow-up, 490 cases of CRC were recorded, and vegetarian diets were related to a decreased incidence of CRC compared to non-vegetarian diets28. Actually, comparing studies is difficult due to the absence of studies on the association between PHDI and CRC, differences in dietary habits of different countries, and differences in the use of various components and cut-off points.
The PHDI recommends increasing the intake of unsaturated oils, nuts, whole grains, legumes, vegetables, and fruits, and limiting the intake of added sugar, starchy vegetables, refined grains, and red and processed meat, which could improve the quality of the diet10,12,29.
Fruits and vegetables, due to their high content of antioxidants and antitumor agents, such as folic acid, vitamins A, C, and E, minerals, selenium, fiber, carotenoids, polyphenols, flavonoids, isothiocyanates, and omega-3 fatty acids, have protective mechanisms against CRC risk. These nutrients stimulate apoptosis and inhibit cell proliferation, thereby preventing tumor growth30,31,32. The protective property of green leafy vegetables against CRC is related to the anti-genotoxic effect of chlorophyll and the anti-carcinogenic effect of folic acid17,33.
Furthermore, the PHDI is a rich-fiber diet due to its high amounts of legumes, nuts, whole grains, vegetables, and fruits and low amounts of animal proteins10. Dietary fibers are associated with a reduced risk of CRC through the following mechanisms. First, dietary fibers reduce the contact of carcinogens with the colon mucosa by decreasing transit time and increasing fecal bulk, thereby reducing the risk of CRC34. Second, dietary fibers reduce insulin-like growth factor-1 (IGF-1) and hyperinsulinemia, both risk factors for CRC28. Third, dietary fibers have beneficial health effects on gut microbiota through the production and fermentation of short-chain fatty acids (SCFA) named propionate, butyrate, and acetate. Butyrate is the main fuel of colonocytes and maintains the integrity of the intestinal barrier. This SCFA reduces systemic inflammation by preventing the entry of lipopolysaccharide (LPS) from the intestinal wall into the blood. Butyrate also reduces cell proliferation and provokes apoptosis by increasing the expression of p57 and p21 proteins and down-regulating of c-MYC, consequently reducing the risk of CRC35,36,37.
As mentioned, unsaturated oils and seafood are also recommended in the PHD. Olive oil phenolic compounds exert their anti-carcinogenic effects by reducing deoxyribonucleic acid (DNA) damage caused by hydrogen peroxide and downregulating the epidermal growth factor receptor38,39. Omega-3 fatty acids found in fish have anti-carcinogenic effects by reducing the expression of cyclooxygenase-2 (COX-2) and preventing the production of inflammatory cytokines40,41.
The strengths of the current research include the novelty of the subject, the application of a valid FFQ, and the adjustment for a large number of confounding variables. While this study presents novel findings, several limitations should be acknowledged. First, the sample size was small. Second, there may be other confounding factors that were not included in the study. Third, using FFQ, which relies on memory, could introduce measurement errors. Finally, selecting participants from hospitals rather than the general population may result in selection bias.
Conclusions
The findings of the current study indicated a reverse relationship between PHDI and total adequacy, moderation, and ratio scores with the odds of CRC. However, it is suggested that more research be performed in this field in the future to confirm the results of this study.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
-
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J. Clin. 71 (3), 209–249 (2021).
Google Scholar
-
Arnold, M. et al. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 66 (4), 683–691 (2017).
Google Scholar
-
Gavrilas, L. I. et al. Plant-derived bioactive compounds in colorectal cancer: insights from combined regimens with conventional chemotherapy to overcome drug-resistance. Biomedicines. 10 (8), 1948 (2022).
Google Scholar
-
Malcomson, F. C. et al. Adherence to the 2018 World Cancer Research Fund/American Institute for Cancer Research Cancer Prevention Recommendations and cancer risk: a systematic review and meta-analysis. Cancer. 129 (17), 2655–2670 (2023).
Google Scholar
-
Veettil, S. K. et al. Role of Diet in Colorectal Cancer incidence: Umbrella Review of Meta-analyses of prospective observational studies. JAMA Netw. Open. 4 (2), e2037341 (2021).
Google Scholar
-
Park, S. Y. et al. Alcohol Intake and Colorectal Cancer Risk in the multiethnic cohort study. Am. J. Epidemiol. 188 (1), 67–76 (2019).
Google Scholar
-
Kim, H. et al. Total calcium, dairy foods and risk of colorectal cancer: a prospective cohort study of younger US women. Int. J. Epidemiol. 52 (1), 87–95 (2022).
Google Scholar
-
Delgado-Gonzalez, P., Garza-Treviño, E. N., de la Garza Kalife, D. A., Quiroz Reyes, A. & Hernández-Tobías, E. A. Bioactive compounds of dietary origin and their influence on colorectal cancer as chemoprevention. Life. 13 (10): 1977 (2023).
-
Wang, M. et al. Inhibition and potential treatment of colorectal cancer by natural compounds via various signaling pathways. Front. Oncol. 12, 956793 (2022).
Google Scholar
-
Willett, W. et al. Food in the Anthropocene: the EAT–Lancet Commission on healthy diets from sustainable food systems. Lancet. 393 (10170), 447–492 (2019).
Google Scholar
-
Dalile, B. et al. The EAT–Lancet reference diet and cognitive function across the life course. Lancet Planet. Health. 6 (9), e749–e759 (2022).
Google Scholar
-
Cacau, L. T. et al. Development and validation of an index based on EAT-Lancet recommendations: the Planetary Health Diet Index. Nutrients. 13 (5), 1698 (2021).
Google Scholar
-
Wang, F. et al. IDDF2021-ABS-0085 Association of healthy and unhealthy plant-based diets with the risk of colorectal cancer overall and by molecular subtypes. Gut. 70 (Suppl 2), A112–A112 (2021).
-
Kim, J. et al. Plant-based dietary patterns defined by a priori indices and colorectal cancer risk by sex and race/ethnicity: the multiethnic cohort study. BMC Med. 20 (1), 1–14 (2022).
Google Scholar
-
Ren, X. et al. Compliance with the EAT-Lancet diet and risk of colorectal cancer: a prospective cohort study in 98,415 American adults. Front. Nutr. 10:1264178 (2023).
-
Berthy, F. et al. Association between adherence to the EAT-Lancet diet and risk of cancer and cardiovascular outcomes in the prospective NutriNet-Santé cohort. Am. J. Clin. Nutr. 116 (4), 980–991 (2022).
Google Scholar
-
Terry, P., Jain, M., Miller, A. B., Howe, G. R. & Rohan, T. E. Dietary intake of folic acid and colorectal cancer risk in a cohort of women. Int. J. Cancer. 97 (6), 864–867 (2002).
Google Scholar
-
Yarmand, S. et al. A healthful plant-based diet can reduce the risk of developing colorectal cancer: case-control study. J. Health Popul. Nutr. 43 (1), 111 (2024).
Google Scholar
-
Kahrizsangi, M. A. et al. Carbohydrate quality indices and colorectal cancer risk: a case-control study. BMC Cancer. 23 (1), 347 (2023).
Google Scholar
-
Jafari, F. et al. Ultra-processed food intake and risk of colorectal cancer: a matched case-control study. Nutr. Cancer. 75 (2), 532–541 (2023).
Google Scholar
-
Mirmiran, P., Esfahani, F. H., Mehrabi, Y., Hedayati, M. & Azizi, F. Reliability and relative validity of an FFQ for nutrients in the Tehran lipid and glucose study. Public Health. Nutr. 13 (5), 654–662 (2010).
Google Scholar
-
Ghaffarpour, M., Houshiar-Rad, A. & Kianfar, H. The manual for household measures, cooking yields factors and edible portion of foods. Tehran: Nashre Olume Keshavarzy. 7 (213), 42–58 (1999).
-
Nutritionist, I. N-squared computing. Silverton: Nutritionist IV (1998).
-
Biernat, E., Stupnicki, R., Lebiedziński, B. & Janczewska, L. Assessment of physical activity by applying IPAQ questionnaire. Phys. Educ. Sport. 52 (2), 83–89 (2008).
-
Djalalinia, S. et al. Protocol design for large–scale cross–sectional studies of surveillance of risk factors of non–communicable diseases in Iran: STEPs 2016. Arch. Iran. Med. 20 (9)(2017).
-
Moazzen, S., van der Sloot, K. W. J. & Bock GHd, Alizadeh, B. Z. Systematic review and meta-analysis of diet quality and colorectal cancer risk: is the evidence of sufficient quality to develop recommendations? Crit. Rev. Food Sci. Nutr. 61 (16), 2773–2782 (2021).
Google Scholar
-
Chen, Z. et al. Dietary patterns and colorectal cancer: results from a Canadian population-based study. Nutr. J. 14 (1), 1–9 (2015).
Google Scholar
-
Orlich, M. J. et al. Vegetarian dietary patterns and the risk of colorectal cancers. JAMA Intern. Med. 175 (5), 767–776 (2015).
Google Scholar
-
Cacau, L. T. et al. Adherence to the planetary health diet index and obesity indicators in the Brazilian longitudinal study of adult health (ELSA-Brasil). Nutrients. 13 (11), 3691 (2021).
Google Scholar
-
Heber, D. Vegetables, fruits and phytoestrogens in the prevention of diseases. J. Postgrad. Med. 50 (2), 145 (2004).
Google Scholar
-
Gavrilas, L. I. et al. The role of bioactive dietary components in modulating miRNA expression in colorectal cancer. Nutrients. 8 (10), 590 (2016).
Google Scholar
-
Nowak, R., Olech, M. & Nowacka, N. Plant polyphenols as chemopreventive agents. Polyphenols Hum. Health Disease 2014:1289–1307 .
-
Tantamango, Y. M., Knutsen, S. F., Beeson, W. L., Fraser, G. & Sabate, J. Foods and food groups associated with the incidence of colorectal polyps: the Adventist Health Study. Nutr. Cancer. 63 (4), 565–572 (2011).
Google Scholar
-
Aune, D. et al. Dietary fibre, whole grains, and risk of colorectal cancer: systematic review and dose-response meta-analysis of prospective studies. Bmj 343 (2011).
-
Sidhu, S. R. K., Kok, C. W., Kunasegaran, T. & Ramadas, A. Effect of plant-based diets on gut microbiota: a systematic review of Interventional studies. Nutrients. 15 (6), 1510 (2023).
Google Scholar
-
Hu, S. et al. The microbe-derived short chain fatty acid butyrate targets miRNA-dependent p21 gene expression in human colon cancer. PloS One. 6 (1), e16221 (2011).
Google Scholar
-
Hu, S., Liu, L., Chang, E. B., Wang, J-Y. & Raufman, J-P. Butyrate inhibits pro-proliferative miR-92a by diminishing c-Myc-induced mir-17-92a cluster transcription in human colon cancer cells. Mol. Cancer. 14 (1), 1–15 (2015).
Google Scholar
-
Terzuoli, E., Giachetti, A., Ziche, M. & Donnini, S. Hydroxytyrosol, a product from olive oil, reduces colon cancer growth by enhancing epidermal growth factor receptor degradation. Mol. Nutr. Food Res. 60 (3), 519–529 (2016).
Google Scholar
-
Gill, C. I. et al. Potential anti-cancer effects of virgin olive oil phenolson colorectal carcinogenesis models in vitro. Int. J. Cancer. 117 (1), 1–7 (2005).
Google Scholar
-
Lee, J. Y., Sim, T-B., Lee, J. & Na, H-K. Chemopreventive and chemotherapeutic effects of fish oil derived omega-3 polyunsaturated fatty acids on colon carcinogenesis. Clin. Nutr. Res. 6 (3), 147–160 (2017).
Google Scholar
-
Llor, X. et al. The effects of fish oil, olive oil, oleic acid and linoleic acid on colorectal neoplastic processes. Clin. Nutr. 22 (1), 71–79 (2003).
Google Scholar
Acknowledgements
We sincerely thank all field investigators, staff, and participants of the present study.
Author information
Authors and Affiliations
Contributions
F.M., S.A., N.A., A.M., M.N. and M.S.; Contributed to writing the first draft. Z.S. and M.N.; Contributed to all data and statistical analysis, and interpretation of data. Z.S. and B.R.; Contributed to the research concept, supervised the work, and revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
Reprints and permissions
About this article
Cite this article
Mohammadi, F., Alijani, S., Abdollahi, N. et al. The association between Planetary Health Diet Index and the risk of colorectal cancer: a case-control study.
Sci Rep 14, 26546 (2024). https://doi.org/10.1038/s41598-024-78197-6
-
Received: 14 November 2023
-
Accepted: 29 October 2024
-
Published: 03 November 2024
-
DOI: https://doi.org/10.1038/s41598-024-78197-6
Keywords
- Planetary Health Diet Index
- Colorectal cancer
- Colorectal neoplasms
- Iranian