Abstract
Diet quality is closely related to the occurrence of gastrointestinal (GI) cancers; however, few studies have investigated the association between the Healthy Eating Index (HEI-2020) and the alternative Mediterranean diet (aMED) scores and GI (GI) cancers. This study aims to assess the association between HEI-2020, aMED scores and GI cancers. Information from a total of 26,320 participants was included in the National Health and Nutrition Examination Survey (NHANES). In our sub-analysis, we focused on participants with complete dietary and health data, specifically assessing the relationship between diet quality and GI cancers outcomes. The 24-hour recall questionnaire was used to collect and assess the participants’ average dietary intake. Diagnoses of GI cancers were based on self-reported medical history confirmed through physician interviews or linked cancer registry data, ensuring diagnostic reliability. Logistic regression models, restricted cubic splines (RCS), subgroup analysis, and interaction methods were employed to fully evaluate the associations between HEI-2020, aMED scores, and GI cancers. Mediation analysis was also conducted to identify potential mediators of this relationship. Even after fully adjusting for potential confounders, participants with high adherence to the HEI-2020-2020 and aMED scores were significantly associated with a reduced risk of GI cancers. Compared to the lowest tertile of HEI-2020, participants in the highest tertile had a 30% reduced risk of GI cancers (OR 0.70, 95% CI 0.50 to 0.98, p = 0.037). Compared to the lowest tertile of aMED scores, participants in the highest tertile had a 37% reduced risk of GI cancers (OR 0.63, 95% CI 0.44 to 0.92, p = 0.014). RCS analysis indicated that both HEI-2020 and aMED scores were significantly associated with GI cancers; however, no significant non-linear relationship was observed. The primary findings confirm that higher adherence to HEI-2020 and aMED scores is associated with a lower risk of GI cancers. These results suggest that maintaining a high-quality diet may play a crucial role in the prevention and management of these cancers. Further research is needed to elucidate the mechanisms and effects of healthy diet management and high-quality diets in the prevention of GI cancers.
Introduction
The global incidence of malignant tumors is on the rise, with a notable increase in gastrointestinal (GI) cancers such as esophageal, gastric, liver, pancreatic and colorectal cancers1. These types of cancer are major contributors to cancer incidence and mortality worldwide, significantly impacting human health. Gastric cancer stands out as one of the primary GI malignancies, ranking as the second leading cause of cancer-related deaths, as reported by the World Health Organization2. Meanwhile, colorectal cancer ranks as the fourth most prevalent cancer globally and the third leading cause of cancer-related mortality3. Studies indicate that GI tumors are influenced by both environmental factors—including smoking, diet, and obesity—and individual factors such as genetic predisposition, age, and gender4. The World Cancer Research Fund/American Institute for Cancer Research suggests that up to 50% of digestive tract cancers could be prevented through lifestyle and behavioral modifications5. Research findings affirm that consuming red and processed meats increases the risk of colorectal cancer6, whereas intake of dairy products and dietary fibers may decrease the risk of GI cancers7,8. Poor dietary habits, characterized by low consumption of fruits and vegetables and high consumption of red meats and fats, are linked to increased oxidative stress and apoptosis, promoting tumor growth and spread9,10.
In recent years, dietary research has shifted from examining individual foods or nutrients to adopting a more comprehensive approach focused on dietary patterns. Dietary pattern analysis explores the combined effects of multiple foods and nutrients, reflecting the complexity of real-world diets11. Compared to studies focused on isolated foods or nutrients, dietary pattern analysis offers a more accurate prediction of disease risk12. Studies have found that both the Healthy Eating Index (HEI-2020) and alternative Mediterranean Diet (aMED) are associated with reduced GI cancers risk. For example, previous studies have demonstrated that high adherence to the Mediterranean diet is associated with a reduced risk of colorectal and gastric cancers13. Similarly, studies on the HEI-2020 have shown associations between high HEI scores and reduced cancer risks, including some GI cancers, due to adherence to dietary guidelines that emphasize fruits, vegetables, and whole grains while limiting processed meats and added sugars14,15. However, comparisons between HEI-2020 and aMED scores remain limited.
HEI-2020, developed by the U.S. Department of Health and Human Services and the USDA, assesses diet quality based on adherence to the Dietary Guidelines for Americans16,17. In contrast, the Mediterranean Diet (MED) scores focus on high intake of vegetables, fruits, whole grains, and olive oil, with minimal red and processed meats, which are linked to lower cancer risk due to antioxidants like polyphenols18,19,20. The aMED adapts this pattern for Western diets, allowing a broader application beyond the Mediterranean context21.
Additionally, biomarkers such as uric acid and albumin may play roles in cancer risk.
Uric acid acts as an antioxidant at low levels but promotes oxidative stress and inflammation at high levels, potentially contributing to tumor development22,23,24. Albumin reflects both nutritional status and systemic inflammation, with low levels associated with higher cancer risk25,26. Healthy dietary patterns, indicated by high HEI-2020 and aMED scores, generally correlate with higher albumin and lower inflammatory markers, potentially aiding cancer prevention27,28.
While the MED is renowned for its health benefits and its role in preventing various chronic diseases, dietary habits and lifestyles differ markedly across populations, underscoring the need for further research into other dietary indices. The HEI-2020, an advanced and comprehensive dietary assessment tool, has yet to be extensively explored in cancer prevention contexts. This study addresses this gap by evaluating the associations between HEI-2020 and the aMED with GI cancer risk, offering new insights into the impacts of these dietary patterns on cancer prevention. Using data from the 2005–2018 National Health and Nutrition Examination Survey (NHANES), the study also examines the potential mediating effects of serum albumin and uric acid. Understanding how these dietary patterns and biomarkers influence GI cancers development can offer valuable insights for cancer prevention and dietary guidance.
Methods
Study population
This study analyzed data from the NHANES spanning 2005 to 2018. NHANES is a comprehensive cross-sectional survey that evaluates the health and nutritional status of both adults and children in the U.S. every two years. The survey uses a multistage stratified sampling method and includes modules on demographics, diet, physical exams, lab tests, and questionnaires. All methods were performed in accordance with the relevant guidelines and regulations. Information on the NHANES database can be accessed on the official website (https://www.cdc.gov/nchs/nhanes/index.htm).
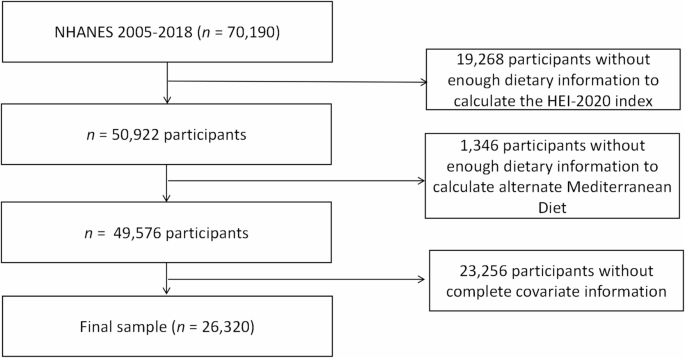
From the data covering seven cycles from 2005 to 2018, a total of 70,190 participants were initially considered. Exclusions were made for 19,268 participants due to incomplete data for calculating the HEI-2020, 1,346 for missing aMED scores, and 23,256 for absent covariate data. Ultimately, 26,320 participants were selected for the study, consisting of 12,722 males and 13,598 females. Additional details are depicted in Fig. 1.

Flowchart for the Study Population: : From NHANES 2005–2018.
Assessment of HEI-2020 and aMED scores
Dietary intake data for this study were obtained from the NHANES database using a 24-hour dietary recall questionnaire administered twice—first through face-to-face interviews and then via telephone within ten days. This approach ensured comprehensive data on the types and quantities of food consumed, along with the times and locations of consumption. The assessments were conducted by trained researchers at Mobile Examination Centers.
The HEI-2020 utilizes data from these dietary recalls combined with the USDA’s food pattern equivalents database. Using a scoring algorithm provided by the National Cancer Institute, the HEI-2020 evaluates diet quality based on thirteen components divided into two categories: adequacy and moderation. Adequacy components, which include vegetables, fruits, whole grains, protein foods, dairy, and healthy fats, reward higher intakes with higher scores. Moderation components, targeting intake of added sugars, sodium, refined grains, and saturated fats, assign higher scores for lower intakes17.
Adherence to the Mediterranean diet was quantified through the aMED scores. This process involved two steps: first, linking the 24-hour dietary recall data to the USDA’s Food Patterns Equivalents Database to standardize food component measurements29. Second, calculating the aMED scores based on intake levels of key food components like fruits, vegetables, red and processed meats, whole grains, alcohol, nuts, legumes, fish, and the ratio of monounsaturated to saturated fatty acids30. For this scoring, intakes of red and processed meats below the median, and moderate alcohol consumption, are each awarded one point; intakes of the other foods above the median also receive one point, with higher aMED scores indicating greater adherence to the MED.
Definition of GI cancers
In this study, GI cancers were identified based on responses to the survey question, “What kind of cancer?” Only responses explicitly indicating the presence of GI tumors were considered valid. The study focused on six types of GI cancers from the NHANES database: esophageal cancer, gastric cancer, liver cancer, pancreatic cancer, colon and rectal cancer. Detailed information on cancer types and tHEI-2020r distribution can be found in Supplementary Table 1. For more detailed information, please refer to the NHANES 2005–2006 MCQ section on the official website (https://wwwn.cdc.gov/Nchs/Nhanes/2005-2006/MCQ_H.htm#MCQ230a).
Assessment of serum albumin and uric acid
In the NHANES study, participants’ fasting serum samples were stored at -30 degrees Celsius and sent to the National Center for Environmental Health. Trained researchers measured serum albumin concentration (g/dL) using the bromocresol purple dye-binding method and determined serum uric acid levels (mg/dL) using the uricase oxidation method. Both measurement processes underwent strict calibration and quality control to ensure accuracy and consistency. More specific details can be seen in https://wwwn.cdc.gov/Nchs/Nhanes/.
Covariates
The study used several covariates such as confounding factors to accurately assess the relationship between diet quality and GI cancers. These included gender, defined as male or female, and age, which was treated as a continuous variable. Other variables included body mass index (BMI) categorized as < 20, 20–25, 25–30, and ≥ 3031; education level categorized as less than high school, high school, and college or above32; race/ethnicity including Mexican American, Non-Hispanic White, Non-Hispanic Black, and Other; alcohol consumption; smoking status; poverty income ratio (PIR) categorized as < 1, 1–3, and ≥ 333; physical activity levels classified as Moderate, Vigorous, and Inactive34; hypertension, and diabetes. PIR refers to the ratio of monthly family income to a poverty threshold specific to family size. Smoking is defined as smoking at the time of the survey or having smoked more than 100 cigarettes in one’s lifetime35. Alcohol consumption is defined as drinking at the time of the survey or having consumed more than 12 alcoholic beverages in one’s lifetime36. Hypertension was identified by a physician-diagnosed condition, elevated average blood pressure (systolic ≥ 130 and/or diastolic ≥ 85 mmHg), or use of antihypertensive medication37. Diabetes was defined by self-reported physician-diagnosed diabetes, use of insulin or hypoglycemic drugs, fasting blood glucose > 126 mg/dL, glycated hemoglobin ≥ 6.5%, or an oral glucose tolerance test result ≥ 200 mg/dL38.
Statistical analysis
The baseline characteristics of participants grouped by HEI-2020 and aMED scores were described using means (standard deviation [SD]) for continuous variables and counts (percentage) for categorical variables. The Wilcoxon rank-sum test and t-test were employed to analyze differences in continuous variables between groups, while the chi-square test was used for categorical variables. The association was evaluated across tertiles of adherence to HEI-2020 and aMED scores. Logistic regression models were developed to examine the association between HEI-2020, aMED scores (as continuous and categorical variables), and GI cancers, reporting odds ratios (ORs) and 95% confidence intervals (CIs). Model 1 adjusted for age, gender, and race. Model 2 included further adjustments for educational level, alcohol consumption, smoking status, PIR, physical activity, hypertension, and diabetes. These variables were selected based on established risk factors for GI cancers identified in previous epidemiological studies. Additionally, we developed the directed acyclic graphs (DAG) to systematically identify potential confounders and guide model adjustment. More specific details can be seen in Supplementary Figs. 1–2. Restricted cubic spline (RCS) models evaluated the dose-response relationship between HEI-2020-2020, aMED, and GI cancers. Mediation analysis was conducted to determine whether serum albumin and uric acid mediated the relationship between dietary quality indices (HEI-2020 and aMED) and GI cancers. The total effect (TE) represents the direct relationship between dietary quality indices and GI cancers, uninfluenced by mediators. The indirect effect (IE) refers to the impact of dietary quality indices on GI cancers through serum albumin and uric acid. The direct effect (DE) represents the impact of dietary quality indices on GI cancers after controlling for levels of serum albumin and uric acid. A significant IE indicates a notable mediation effect, calculated as IE/TE*100%. Additionally, subgroup analyses were conducted based on age, gender, race, educational level, BMI, PIR, hypertension, and diabetes, and interactions were assessed using the p-values of product terms between HEI-2020, aMED, and each stratifying factor.
All statistical analyses and graphics were performed using the R Project for Statistical Computing (version 4.2.3) and RStudio. All tests were two-sided, and a p-value < 0.05 was considered statistically significant.
Results
Baseline characteristics
The study analyzed 26,320 participants, with demographic characteristics presented in Tables 1 and 2. Participants’ HEI-2020 were categorized into tertiles: Tertile 1 (0-45.39), Tertile 2 (45.39–56.17), and Tertile 3 (56.17–94.19)16, while their aMED scores were similarly grouped into tertiles: Tertile 1 (0–4), Tertile 2 (4–5), and Tertile 3 (≥ 6). Participants in the highest tertile of adherence to HEI-2020 and aMED were more likely to be female, older, have a BMI ≥ 20, hold a college degree or higher, belong to other races, be drinkers and smokers, have a PIR > 3, have hypertension, and lower uric acid levels compared to those in the lowest tertiles. Additionally, those in the highest HEI-2020 tertile were more likely to engage in moderate physical activity, have diabetes, and higher albumin levels, while those in the highest aMED tertile were more likely to engage in vigorous physical activity.
Association between HEI-2020, aMED scores and GI cancers
As shown in Table 3, in Model 2, continuous HEI-2020 continued to show a significant negative correlation with the risk of GI cancers (OR 0.84, 95% CI 0.71 to 0.98, p = 0.028). Compared with the lowest tertile of adherence to the HEI-2020, the highest tertile was associated with a 30% lower risk of GI cancers (OR = 0.70, 95% CI: 0.50 to 0.98, p = 0.037).
As shown in Supplementary Table 2, in Model 2, in the female subgroup, the continuous HEI-2020 was significantly negatively correlated with the risk of GI cancers (OR 0.99, 95% CI 0.97 to 1.00, p = 0.030). In males, the continuous HEI-2020 was significantly negatively correlated with the risk of GI cancers (OR 0.98, 95% CI 0.97 to 1.00, p = 0.020). Compared with the lowest tertile of adherence to the HEI-2020, the highest tertile was associated with a statistically significant 35% reduction in GI cancers risk (OR = 0.65, 95% CI: 0.42–1.00, p = 0.050).
Participants were also grouped by aMED scores into tertiles: Tertile 1 (aMED scores 0–4), Tertile 2 (aMED scores 4–5), and Tertile 3 (aMED scores ≥ 6). In Model 2, the continuous aMED scores was also significantly negatively correlated with the risk of GI cancers (OR 0.80, 95% CI 0.67 to 0.96, p = 0.014), compared with the lowest tertile of adherence to the aMED scores, the highest tertile was associated with a 37% lower risk of GI cancers (OR 0.63, 95% CI 0.44 to 0.92, p = 0.014).
As shown in Supplementary Table 3, in Model 2, for females, the continuous aMED scores was significantly negatively correlated with the risk of GI cancers (OR 0.83, 95% CI 0.68 to 1.00, p = 0.050). Compared with the lowest tertile of adherence to the aMED scores, the highest tertile was associated with a 32% lower risk of GI cancers (OR 0.68, 95% CI 0.41 to 0.98, p = 0.020). For males group, the continuous aMED scores was also significantly negatively correlated with the risk of GI cancers (OR 0.83, 95% CI 0.69 to 1.00, p = 0.050).
Nonlinearity analysis using RCS
This study utilized an RCS model to analyze the dose-response relationship between dietary quality indices and GI cancers, setting knots at the 5th, 35th, 65th, and 95th percentiles, with the 5th percentile as the reference point. The multivariable model was adjusted using the same covariates as previous analyses. Results indicated a significant overall association between both HEI-2020 and GI cancers and aMED and GI cancers; however, the nonlinearity in these relationships was not significant (HEI-2020: p for overall association = 0.036, p for nonlinearity = 0.723; aMED: p for overall association = 0.033, p for nonlinearity = 0.742). Detailed visual representations are provided in Fig. 2.

The Association between HEI-2020, aMED Score, and Gastrointestinal Cancers Using the Restricted Cubic Spline Regression Model. (A), HEI-2020; (B), aMED Score. Graphs show ORs for end according to HEI-2020, aMED Score adjusted for sex, age, ethnicity, education level, drink, smoke, PIR, Hypertension, Diabetes. Data were fitted by a logistic regression model, and the model was conducted with 4 knots at the 5th, 35th, 65th, 95th percentiles of HEI-2020, aMED Score (reference is the 5th percentile). Solid lines indicate ORs, and shadow shape indicate 95% CIs. HEI, Healthy Eating Index; aMED, Alternative Mediterranean Diet; OR, odds ratio; CI, confidence interval.
Subgroup analysis
Subgroup analyses revealed a consistently negative correlation between HEI-2020 scores and GI cancer risk across various demographics. Notably, as shown in Fig. 3A, stronger negative correlations were observed in subgroups aged over 60 years (OR 0.83, 95% CI 0.69 to 0.99), Non-Hispanic Whites (OR 0.82, 95% CI 0.67 to 1.00), those with a college education or higher (OR 0.76, 95% CI 0.61 to 0.95), PIR between 1 and 3 (OR 0.78, 95% CI 0.62 to 0.98), individuals with hypertension (OR 0.75, 95% CI 0.62 to 0.91), and non-diabetics (OR 0.81, 95% CI 0.67 to 0.98). No interaction effects were detected regarding age, gender, race, education level, BMI, PIR, hypertension, or diabetes.

Subgroup Analyses of the Association between HEI-2020, aMED Score, and Gastrointestinal Cancers. (A), HEI-2020; (B), aMED Score. HEI, Healthy Eating Index; aMED, Alternative Mediterranean Diet;
Similarly, as shown in Fig. 3B, a negative correlation between aMED Scores and GI cancer risk was consistent across all subgroups, with more pronounced effects among those with a college education or higher (OR 0.80, 95% CI 0.67 to 0.96), a BMI of 25–30 (OR 0.78, 95% CI 0.64 to 0.96), a PIR of 1–3 (OR 0.81, 95% CI 0.67 to 0.98), hypertensive individuals (OR 0.85, 95% CI 0.73 to 1.00), and non-diabetics (OR 0.82, 95% CI 0.71 to 0.96). Again, no interaction effects were observed.
Mediation analysis
The study conducted a mediation analysis to investigate whether serum uric acid and albumin levels mediate the relationship between dietary quality indices and GI cancer risk. The analysis used HEI-2020 and aMED Scores as independent variables, GI cancers as the dependent variable, and serum uric acid and albumin as mediators.
For HEI-2020, serum uric acid levels exhibited a significant mediating effect, accounting for 1.71% of the association. The IE was − 0.00002 (95% CI: -0.00003 to -0.00000), as depicted in Fig. 4A. For aMED scores, uric acid levels also showed a significant mediating role, representing 3.13% of the association, with an IE of -0.0001 (95% CI: -0.0002 to -0.0000), detailed in Fig. 4C.

Mediation Analyses of the Association between HEI-2020, aMED Score and Gastrointestinal Cancers. (A), serum uric acid, (B), serum albumin partially mediates the relationship between HEI-2020 and GI Cancers; (C), serum uric acid, (D), serum albumin partially mediates the relationship between aMED Score and GI Cancers; HEI, Healthy Eating Index; aMED, Alternative Mediterranean Diet;
Regarding HEI-2020, serum albumin levels had a notable mediating effect, explaining 14.29% of the relationship, with an IE of -0.0001 (95% CI: -0.0002 to -0.0000), as shown in Fig. 4B. Similarly, for aMED scores, albumin levels significantly mediated the relationship, accounting for 22.58% of the association, with an IE of -0.0001 (95% CI: -0.0003 to -0.0000), illustrated in Fig. 4D. Detailed representations of these mediating effects are provided in Fig. 4.
Discussion
This study utilized data from NHANES 2005–2018 to examine the relationships between HEI-2020, aMED scores, and GI cancers. The findings indicate that higher HEI-2020 and aMED scores are significantly associated with reduced risks of GI cancers. Mediation analyses suggest that serum albumin and uric acid levels partially mediate these relationships. Subgroup analyses and interaction results reinforce the consistency of these effects across different genders, races, ages, educational levels, BMIs, hypertension, and diabetes, enhancing the reliability of our conclusions.
GI cancers pose a growing global health challenge3. Identifying modifiable risk factors, such as diet quality, is crucial for effective prevention and management strategies. Although the MED is renowned for its health benefits, dietary patterns vary widely across different populations, underscoring the need for further research into other dietary indices. This study contributes new insights into how dietary quality affects GI cancer risks and the roles of serum albumin and uric acid as mediators. These findings highlight the importance of diet quality in cancer prevention and can inform personalized dietary guidance and public health policies.
Our results align with previous studies emphasizing the benefits of high dietary quality in preventing GI cancers39. For instance, a systematic review associated high-quality diets with reduced risks of upper digestive tract cancers40. Furthermore, studies like the Newfoundland study and the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort study have demonstrated that diets rich in anti-inflammatory components correlate with lower cancer incidences10,41. These studies collectively support the notion that enhancing diet quality can significantly mitigate cancer risks. In existing literature, individuals with higher HEI and MED scores generally exhibit a lower risk of GI cancers, particularly when consuming adequate amounts of vegetables, fruits, whole grains, and olive oil13,42,43. These studies collectively support the notion that improving diet quality can substantially reduce cancer risk.
Both HEI-2020 and aMED scores prioritize the intake of fruits and vegetables, known for tHEI-2020r rich antioxidant and anti-inflammatory properties. Antioxidants, such as vitamin C, vitamin E, and β-carotene and polyphenolic compounds, help reduce free radical formation and oxidative DNA damage and suppress inflammatory responses, thus lowering cancer risk44. Anti-inflammatory components can mitigate chronic inflammation, further decreasing tumor risks45. Moreover, these dietary indices advocate for reduced intake of red and processed meats, known for their association with higher cancer risks46, and suggest alternative protein sources like fish, legumes, and nuts47. Reducing intake of nitrates and other carcinogens commonly found in these meats is also emphasized to decrease cancer risks48.
Compared to aMED scores, the relationship between HEI-2020 scores and the risk of digestive tract tumors offers significant protection in certain subgroups; however, the overall impact of aMED scores is less pronounced than that of HEI-2020. When analyzing the two dietary indices both as continuous variables and in tertiles through logistic regression, the protective effect of HEI-2020 is found to be more consistent and pronounced across all levels. This consistency may stem from HEI-2020’s inclusion of a wider array of food categories and nutrients, which enables a more thorough evaluation of dietary quality. The breadth and depth of HEI-2020 facilitate a more reliable reflection of dietary quality changes at various levels, thereby revealing a stronger and more consistent protective effect in the highest tertile. This effect should be credited to the comprehensive nature of HEI-2020, alongside considerations of sample size and statistical power, and its superior capability in assessing dietary quality comprehensively49. In comparison, while aMED also exhibits significant protective effects in the highest tertile, its consistency across different levels does not match that of HEI-2020, leading to a more pronounced protective impact of HEI-2020 against digestive tract tumors50.
Our mediation analysis reveals that serum albumin plays a significant role in mediating the relationship between HEI-2020 scores and GI cancers, accounting for 14.29% of the total effect25. As an acute-phase protein, albumin levels typically decrease during inflammation and oxidative stress, which are conditions often mitigated by a healthy diet rich in protein. This helps maintain higher serum albumin levels, reducing inflammation and oxidative stress. Conversely, uric acid, a byproduct of purine metabolism, is generally linked to higher risks of metabolic syndrome, cardiovascular diseases, and certain cancers51,52. It is known to contribute to oxidative stress by activating enzymes like NADPH oxidase, which promotes the production of reactive oxygen species and stimulates tumor cell proliferation53. These dietary patterns, which recommend high dietary fiber, low fat, and rich antioxidants, help limit uric acid production, thereby aiding in the reduction of inflammation and oxidative stress and potentially inhibiting tumor growth. These findings underscore the importance of comprehensive dietary assessments and their potential implications in understanding the mechanisms behind GI tumor development. Future research should focus on elucidating the complex interactions between healthy dietary patterns and these mediating factors, enhancing our strategies for the prevention and management of GI tumors.
The subgroup analysis results indicate that the protective effect of HEI-2020 on gastrointestinal cancer is more pronounced in men, while it does not reach statistical significance in women. This difference may be attributed to physiological factors, including the effects of sex hormones (particularly estrogen) and variations in oxidative stress and antioxidant capacity. First, estrogen plays a significant antioxidant and anti-inflammatory role in women, helping reduce oxidative stress and regulate the production of pro-inflammatory cytokines54. This protective effect may partially substitute for the antioxidant benefits of a high-quality diet, potentially leading to a reduced additional benefit from dietary improvements in women55. Furthermore, studies have shown that women generally exhibit higher activity levels of antioxidant enzymes, such as superoxide dismutase (SOD) and glutathione peroxidase (GPx), compared to men, which further enhances their resistance to oxidative stress56.
This study leverages the HEI-2020, a comprehensive tool reflecting the “2020–2025 Dietary Guidelines for Americans.” It covers a broad spectrum of food categories and nutrients, such as fruits, vegetables, whole grains, proteins, dairy, healthy fats, added sugars, and sodium, enabling a detailed assessment of diet quality57. The use of an RCS model to examine the dose-response relationship between diet quality and GI tumors reveals a significant negative correlation, although nonlinearity in this relationship warrants further study. Additionally, this research initially explores the mediating roles of serum albumin and uric acid, suggesting these biomarkers could be critical pathways through which diet quality impacts the development and progression of GI tumors. The data sourced from the nationally representative NHANES database enhances the robustness and generalizability of our findings, strengthening the potential impact on public health.
This study still has some limitations. First, the cross-sectional design limits the ability to establish causality between dietary quality and GI tumor risk. While there is a significant association between higher diet quality and reduced tumor risk, the direct influence of diet on tumor development remains unclear. Future research should include prospective cohort studies to assess these temporal relationships more accurately. A key methodological consideration pertains to the inherent limitations of NHANES dietary assessment. While the survey employs the validated USDA Automated Multiple-Pass Method, the 24-hour dietary recall methodology is subject to potential measurement error and various forms of bias58,59. These include recall bias, social desirability bias in reporting perceived healthy or unhealthy foods, and challenges in portion size estimation60. Although NHANES implements a second 24-hour recall for a subset of participants to account for within-person variation, this approach may incompletely capture long-term dietary patterns relevant to cancer risk. Nevertheless, validation studies have demonstrated reasonable correlations between NHANES dietary recalls and objective biomarkers for specific nutrients, supporting the utility of these data for population-level dietary assessment61. Moreover, despite adjusting for various factors like age, gender, race, education level, alcohol consumption, smoking status, PIR, physical activity, hypertension, and diabetes in our analyses, other potential confounders such as family history, genetic predispositions, and gut microbiome interactions remain unexplored. Addressing these in future studies through polygenic risk scoring, metabolomics and proteomics could provide a more comprehensive understanding of the intricate links between diet quality and GI tumors.
To strengthen future research in this area, we recommend combining self-reported dietary data with objective measures such as nutritional biomarkers, implementing more frequent dietary assessments, and utilizing emerging technologies for dietary assessment that may reduce recall bias. Furthermore, validating our findings in prospective cohort studies with longer follow-up periods would help establish the temporal relationship between dietary patterns and GI cancer risk more definitively.
Conclusion
This study underscores the significance of diet quality, as assessed by HEI-2020 and aMED scores, in the prevention and management of GI cancers. The HEI-2020, with its comprehensive dietary evaluation, demonstrates potential in digestive tract tumor prevention and warrants further research. Healthcare providers and public health initiatives should enhance awareness around diet quality, as well as develop refined dietary assessment tools to uncover further preventive capabilities. Given diverse dietary habits across cultures, tailoring recommendations to various ethnic groups poses challenges that may impact compliance. Future health education efforts must promote healthy eating practices through customized strategies. Additionally, longer follow-ups and larger cohorts in future studies would strengthen these findings, while exploring the mechanisms of dietary impact on tumor risks could guide tailored dietary guidelines and policies.
Data availability
This study used data from the National Health and Nutrition Examination Survey (NHANES) (https://www.cdc.gov/nchs/nhanes/index.htm).
References
-
Jardim, S. R., de Souza, L. M. P. & de Souza, H. S. P. The rise of gastrointestinal cancers as a global phenomenon: unhealthy behavior or progress? Int. J. Environ. Res. Public. Health 20. https://doi.org/10.3390/ijerph20043640 (2023).
-
Wang, S. et al. Global, regional, and national lifetime risks of developing and dying from gastrointestinal cancers in 185 countries: a population-based systematic analysis of GLOBOCAN. Lancet Gastroenterol. Hepatol. 9, 229–237. https://doi.org/10.1016/s2468-1253(23)00366-7 (2024).
Google Scholar
-
Vernia, F., Longo, S., Stefanelli, G., Viscido, A. & Latella, G. Dietary factors modulating colorectal carcinogenesis. Nutrients 13. https://doi.org/10.3390/nu13010143 (2021).
-
Keum, N. & Giovannucci, E. Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat. Rev. Gastroenterol. Hepatol. 16, 713–732. https://doi.org/10.1038/s41575-019-0189-8 (2019).
Google Scholar
-
Fund, W. C. R. & Research (2018). A. I. o. C.
-
Farvid, M. S. et al. Consumption of red meat and processed meat and cancer incidence: a systematic review and meta-analysis of prospective studies. Eur. J. Epidemiol. 36, 937–951. https://doi.org/10.1007/s10654-021-00741-9 (2021).
Google Scholar
-
Aune, D. et al. Dairy products and colorectal cancer risk: a systematic review and meta-analysis of cohort studies. Annals Oncology: Official J. Eur. Soc. Med. Oncol. 23, 37–45. https://doi.org/10.1093/annonc/mdr269 (2012).
Google Scholar
-
Ma, Y. et al. Dietary fiber intake and risks of proximal and distal colon cancers: a meta-analysis. Medicine 97, e11678. https://doi.org/10.1097/md.0000000000011678 (2018).
Google Scholar
-
Tangestani, H., Salari-Moghaddam, A., Ghalandari, H. & Emamat, H. Adherence to the Dietary approaches to stop hypertension (DASH) dietary pattern reduces the risk of colorectal cancer: a systematic review and meta-analysis. Clin. Nutr. 39, 2975–2981. https://doi.org/10.1016/j.clnu.2020.02.002 (2020).
Google Scholar
-
Sharma, I. et al. Inflammatory diet and risk for colorectal cancer: a population-based case-control study in Newfoundland. Can. Nutr. 42, 69–74. https://doi.org/10.1016/j.nut.2017.05.010 (2017).
Google Scholar
-
Hu, F. B. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr. Opin. Lipidol. 13, 3–9. https://doi.org/10.1097/00041433-200202000-00002 (2002).
Google Scholar
-
Nestel, P. J., Beilin, L. J. & Mori, T. A. Changing dietary approaches to prevent cardiovascular disease. Curr. Opin. Lipidol. 31, 313–323. https://doi.org/10.1097/mol.0000000000000709 (2020).
Google Scholar
-
Schwingshackl, L., Schwedhelm, C., Galbete, C. & Hoffmann, G. Adherence to Mediterranean Diet and Risk of Cancer: an updated systematic review and Meta-analysis. Nutrients 9. https://doi.org/10.3390/nu9101063 (2017).
-
Steck, S. E., Guinter, M., Zheng, J. & Thomson, C. A. Index-based dietary patterns and colorectal cancer risk: a systematic review. Adv. Nutr. 6, 763–773. https://doi.org/10.3945/an.115.009746 (2015).
Google Scholar
-
Li, W. Q. et al. Index-based dietary patterns and risk of esophageal and gastric cancer in a large cohort study. Clin. Gastroenterol. Hepatology: Official Clin. Pract. J. Am. Gastroenterological Association 11, 1130–1136e1132. https://doi.org/10.1016/j.cgh.2013.03.023 (2013).
Google Scholar
-
Luo, T. & Tseng, T. S. Diet quality as assessed by the healthy eating index-2020 among different smoking status: an analysis of national health and nutrition examination survey (NHANES) data from 2005 to 2018. BMC Public. Health 24, 1212. https://doi.org/10.1186/s12889-024-18630-7 (2024).
Google Scholar
-
Krebs-Smith, S. M. et al. Update of the healthy eating index: HEI-2015. J. Acad. Nutr. Diet. 118, 1591–1602. https://doi.org/10.1016/j.jand.2018.05.021 (2018).
Google Scholar
-
Mentella, M. C., Scaldaferri, F., Ricci, C., Gasbarrini, A. & Miggiano, G. A. D. Cancer and Mediterranean Diet: a review. Nutrients 11. https://doi.org/10.3390/nu11092059 (2019).
-
Farinetti, A., Zurlo, V., Manenti, A., Coppi, F. & Mattioli, A. V. Mediterranean diet and colorectal cancer: a systematic review. Nutrition 43–44, 83–88. https://doi.org/10.1016/j.nut.2017.06.008 (2017).
Google Scholar
-
Yammine, A. et al. Polyphenols of the Mediterranean Diet and their metabolites in the Prevention of Colorectal Cancer. Molecules 26. https://doi.org/10.3390/molecules26123483 (2021).
-
Wang, Y., Fan, H., Ren, Z., Liu, X. & Niu, X. Sleep disorder, Mediterranean diet, and all-cause and cause-specific mortality: a prospective cohort study. BMC Public. Health 23, 904. https://doi.org/10.1186/s12889-023-15870-x (2023).
Google Scholar
-
Sautin, Y. Y. & Johnson, R. J. Uric acid: the oxidant-antioxidant paradox. Nucleosides Nucleotides Nucleic Acids 27, 608–619. https://doi.org/10.1080/15257770802138558 (2008).
Google Scholar
-
Kanellis, J. & Kang, D. H. Uric acid as a mediator of endothelial dysfunction, inflammation, and vascular disease. Semin Nephrol. 25, 39–42. https://doi.org/10.1016/j.semnephrol.2004.09.007 (2005).
Google Scholar
-
Choi, H. K. & Curhan, G. Gout: epidemiology and lifestyle choices. Curr. Opin. Rheumatol. 17, 341–345 (2005).
Google Scholar
-
Don, B. R. & Kaysen, G. Serum albumin: relationship to inflammation and nutrition. Semin. Dial. 17, 432–437. https://doi.org/10.1111/j.0894-0959.2004.17603.x (2004).
Google Scholar
-
Gupta, D. & Lis, C. G. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr. J. 9, 69. https://doi.org/10.1186/1475-2891-9-69 (2010).
Google Scholar
-
Kuczmarski, M. F. & Weddle, D. O. Position paper of the American Dietetic Association: nutrition across the spectrum of aging. J. Am. Diet. Assoc. 105, 616–633. https://doi.org/10.1016/j.jada.2005.02.026 (2005).
Google Scholar
-
Estruch, R. et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet supplemented with Extra-virgin Olive oil or nuts. N Engl. J. Med. 378, e34. https://doi.org/10.1056/NEJMoa1800389 (2018).
Google Scholar
-
Fan, Y. et al. Non-linear association between Mediterranean diet and depressive symptom in U.S. adults: a cross-sectional study. Front. Psychiatry. 13, 936283. https://doi.org/10.3389/fpsyt.2022.936283 (2022).
Google Scholar
-
Fung, T. T. et al. Diet-quality scores and plasma concentrations of markers of inflammation and endothelial dysfunction. Am. J. Clin. Nutr. 82, 163–173. https://doi.org/10.1093/ajcn.82.1.163 (2005).
Google Scholar
-
Powell-Wiley, T. M. et al. Obesity and Cardiovascular Disease: A Scientific Statement from the American Heart Association. Circulation 143, e984–e1010. https://doi.org/10.1161/cir.0000000000000973 (2021).
Google Scholar
-
Yeom, H. E. & Lee, J. The association of education level with autonomy support, self-efficacy and health behaviour in patients with cardiovascular risk factors. J. Clin. Nurs. 31, 1547–1556. https://doi.org/10.1111/jocn.16008 (2022).
Google Scholar
-
Odutayo, A. et al. Income disparities in Absolute Cardiovascular Risk and Cardiovascular Risk factors in the United States, 1999–2014. JAMA Cardiol. 2, 782–790. https://doi.org/10.1001/jamacardio.2017.1658 (2017).
Google Scholar
-
Chang, Y., Yu, C., Dai, X., Sun, H. & Tang, T. Association of dietary inflammatory index and dietary oxidative balance score with gastrointestinal cancers in NHANES 2005–2018. BMC Public. Health 24, 2760. https://doi.org/10.1186/s12889-024-20268-4 (2024).
Google Scholar
-
Wang, X. et al. Association of dietary inflammatory potential, dietary oxidative balance score and biological aging. Clin. Nutr. 43, 1–10. https://doi.org/10.1016/j.clnu.2023.11.007 (2024).
Google Scholar
-
Qiu, X., Sun, X., Li, H. O., Wang, D. H. & Zhang, S. M. Maternal alcohol consumption and risk of postpartum depression: a meta-analysis of cohort studies. Public. Health 213, 163–170. https://doi.org/10.1016/j.puhe.2022.08.020 (2022).
Google Scholar
-
Plante, T. B. et al. Life’s simple 7 and Incident Hypertension: the REGARDS Study. J. Am. Heart Association. 9, e016482. https://doi.org/10.1161/jaha.120.016482 (2020).
Google Scholar
-
Diagnosis and classification of diabetes mellitus. Diabetes care 34(Suppl 1), 62–69. https://doi.org/10.2337/dc11-S062 (2011).
-
Buckland, G. et al. Adherence to a Mediterranean diet and risk of gastric adenocarcinoma within the European prospective investigation into Cancer and Nutrition (EPIC) cohort study. Am. J. Clin. Nutr. 91, 381–390. https://doi.org/10.3945/ajcn.2009.28209 (2010).
Google Scholar
-
Moazzen, S., van der Sloot, K. W. J., Vonk, R. J., de Bock, G. H. & Alizadeh, B. Z. Diet Quality and Upper gastrointestinal cancers risk: a Meta-analysis and critical Assessment of evidence quality. Nutrients 12. https://doi.org/10.3390/nu12061863 (2020).
-
La Vecchia, C. & Bosetti, C. Diet and cancer risk in Mediterranean countries: open issues. Public. Health Nutr. 9, 1077–1082. https://doi.org/10.1017/s1368980007668475 (2006).
Google Scholar
-
Morze, J. et al. An updated systematic review and meta-analysis on adherence to mediterranean diet and risk of cancer. Eur. J. Nutr. 60, 1561–1586. https://doi.org/10.1007/s00394-020-02346-6 (2021).
Google Scholar
-
Julián-Serrano, S., Reedy, J., Robien, K. & Stolzenberg-Solomon, R. Adherence to 5 Diet Quality indices and Pancreatic Cancer Risk in a large US prospective cohort. Am. J. Epidemiol. 191, 1584–1600. https://doi.org/10.1093/aje/kwac082 (2022).
Google Scholar
-
Valko, M., Rhodes, C. J., Moncol, J., Izakovic, M. & Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 160, 1–40. https://doi.org/10.1016/j.cbi.2005.12.009 (2006).
Google Scholar
-
Aggarwal, B. B. & Sung, B. Pharmacological basis for the role of curcumin in chronic diseases: an age-old spice with modern targets. Trends Pharmacol. Sci. 30, 85–94. https://doi.org/10.1016/j.tips.2008.11.002 (2009).
Google Scholar
-
Chan, D. S. et al. Red and processed meat and colorectal cancer incidence: meta-analysis of prospective studies. PloS One 6, e20456. https://doi.org/10.1371/journal.pone.0020456 (2011).
Google Scholar
-
Eichelmann, F., Schwingshackl, L., Fedirko, V. & Aleksandrova, K. Effect of plant-based diets on obesity-related inflammatory profiles: a systematic review and meta-analysis of intervention trials. Obes. Rev. 17, 1067–1079. https://doi.org/10.1111/obr.12439 (2016).
Google Scholar
-
Bastide, N. M., Pierre, F. H. & Corpet, D. E. Heme iron from meat and risk of colorectal cancer: a meta-analysis and a review of the mechanisms involved. Cancer Prev. Res. (Phila) 4, 177–184. https://doi.org/10.1158/1940-6207.Capr-10-0113 (2011).
Google Scholar
-
Reedy, J. et al. Higher diet quality is associated with decreased risk of all-cause, cardiovascular disease, and cancer mortality among older adults. J. Nutr. 144, 881–889. https://doi.org/10.3945/jn.113.189407 (2014).
Google Scholar
-
Sofi, F., Abbate, R., Gensini, G. F. & Casini, A. Accruing evidence on benefits of adherence to the Mediterranean diet on health: an updated systematic review and meta-analysis. Am. J. Clin. Nutr. 92, 1189–1196. https://doi.org/10.3945/ajcn.2010.29673 (2010).
Google Scholar
-
Zhu, Y., Pandya, B. J. & Choi, H. K. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007–2008. Arthritis Rheum. 63, 3136–3141. https://doi.org/10.1002/art.30520 (2011).
Google Scholar
-
Jiang, M., Ren, L., Chen, S. & Li, G. Serum uric acid levels and risk of eight site-specific cancers: a mendelian randomization study. Front. Genet. 12, 608311. https://doi.org/10.3389/fgene.2021.608311 (2021).
Google Scholar
-
Sautin, Y. Y., Nakagawa, T., Zharikov, S. & Johnson, R. J. Adverse effects of the classic antioxidant uric acid in adipocytes: NADPH oxidase-mediated oxidative/nitrosative stress. Am. J. Physiol. Cell. Physiol. 293, C584–596. https://doi.org/10.1152/ajpcell.00600.2006 (2007).
Google Scholar
-
Allegra, A., Caserta, S., Genovese, S., Pioggia, G. & Gangemi, S. Gender differences in oxidative stress in relation to Cancer susceptibility and survival. Antioxid. (Basel Switzerland) 12. https://doi.org/10.3390/antiox12061255 (2023).
-
Tiberi, J. et al. Sex differences in antioxidant defence and the regulation of redox homeostasis in physiology and pathology. Mech. Ageing Dev. 211, 111802. https://doi.org/10.1016/j.mad.2023.111802 (2023).
Google Scholar
-
Kim, S. Y. Oxidative stress and gender disparity in cancer. Free Radic. Res. 56, 90–105. https://doi.org/10.1080/10715762.2022.2038789 (2022).
Google Scholar
-
Shams-White, M. M. et al. Healthy eating Index-2020: review and update process to reflect the Dietary guidelines for Americans,2020–2025. J. Acad. Nutr. Diet. 123, 1280–1288. https://doi.org/10.1016/j.jand.2023.05.015 (2023).
Google Scholar
-
Stote, K. S., Radecki, S. V., Moshfegh, A. J., Ingwersen, L. A. & Baer, D. J. The number of 24 h dietary recalls using the US Department of Agriculture’s automated multiple-pass method required to estimate nutrient intake in overweight and obese adults. Public Health. Nutr. 14, 1736–1742. https://doi.org/10.1017/s1368980011000358 (2011).
Google Scholar
-
Ahluwalia, N., Dwyer, J., Terry, A., Moshfegh, A. & Johnson, C. Update on NHANES Dietary Data: Focus on Collection, Release, Analytical considerations, and uses to inform Public Policy. Adv. Nutr. 7, 121–134. https://doi.org/10.3945/an.115.009258 (2016).
Google Scholar
-
Fanelli Kuczmarski, M. et al. Aspects of Dietary Diversity Differ in Their Association with Atherosclerotic Cardiovascular Risk in a racially diverse US Adult Population. Nutrients 11. https://doi.org/10.3390/nu11051034 (2019).
-
Jun, S. et al. Association of food insecurity with dietary intakes and nutritional biomarkers among US children, National Health and Nutrition Examination Survey (NHANES) 2011–2016. Am. J. Clin. Nutr. 114, 1059–1069. https://doi.org/10.1093/ajcn/nqab113 (2021).
Google Scholar
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Writing – original draft preparation: W.W., Y.C.; Writing – review and editing: G.C.; Conceptualization: G.C.; Methodology: Y.C.; Formal analysis and investigation: W.W., Y.C.; Funding acquisition: G.C.; Resources: W.W.; Supervision: G.C., and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The National Health and Nutrition Examination Survey involving human participants was reviewed and approved by the National Center for Health Statistics Ethics Review Board. The patients/participants provided their written informed consent. This study involved secondary data analysis of the National Health and Nutrition Examination Survey, and this study we conducted was exempt from institutional review for this reason.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
Reprints and permissions
About this article
Cite this article
Wang, W., Chang, Y. & Chen, G. Association between Healthy Eating Index-2020, alternative Mediterranean Diet scores, and gastrointestinal cancer risk in NHANES 2005–2018.
Sci Rep 15, 3983 (2025). https://doi.org/10.1038/s41598-025-88317-5
-
Received: 11 July 2024
-
Accepted: 28 January 2025
-
Published: 01 February 2025
-
DOI: https://doi.org/10.1038/s41598-025-88317-5
Keywords
- Healthy Eating Index (HEI-2020)
- Alternative Mediterranean Diet (aMED)
- GI cancers
- National Health and Nutrition Examination Survey (NHANES)
- Mediation analysis